3. Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 2006;355:685–94.


4. Jeng SF, Hsu CH, Tsao PN, Chou HC, Lee WT, Kao HA, et al. Bronchopulmonary dysplasia predicts adverse developmental and clinical outcomes in very-low-birthweight infants. Dev Med Child Neurol 2008;50:51–7.


5. Grier DG, Halliday HL. Corticosteroids in the prevention and management of bronchopulmonary dysplasia. Semin Neonatol 2003;8:83–91.


6. Sharp M, DeMauro SB. Counterbalanced comparison of the BSID-II and Bayley-III at eighteen to twenty-two months corrected age. J Dev Behav Pediatr 2017;38:322–9.


7. Johnson S, Moore T, Marlow N. Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatr Res 2014;75:670–4.


8. Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006;117:1253–61.


9. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–9.


11. Guerra CC, Barros MC, Goulart AL, Fernandes LV, Kopelman BI, Santos AM. Premature infants with birth weights of 1500-1999 g exhibit considerable delays in several developmental areas. Acta Paediatr 2014;103:e1–6.


12. Chang YS, Ahn SY, Park WS. The establishment of the Korean Neonatal Network (KNN). Neonatal Med 2013;20:169–78.

13. The Executive Committee of Korean Neonatal Network. 2016 Korean Neonatal Network Annual Report. Cheongju:Korean Centers for Disease Control and Prevention, 2017, pp 4–63.
14. The Executive Committee of Korean Neonatal Network. 2020 Korean Neonatal Network Annual Report. Cheongju:Korean Centers for Disease Control and Prevention, 2011, pp 4–68.
15. The Executive Committee of Korean Neonatal Network. 2017 Korean Neonatal Network Annual Report. Cheongju:Korean Centers for Disease Control and Prevention, 2018, pp 4–65.
16. The Executive Committee of Korean Neonatal Network. 2018 Korean Neonatal Network Annual Report. Cheongju:Korean Centers for Disease Control and Prevention, 2021, pp 4–68.
17. The Executive Committee of Korean Neonatal Network. 2019 Korean Neonatal Network Annual Report. Cheongju:Korean Centers for Disease Control and Prevention, 2021, pp 4–68.
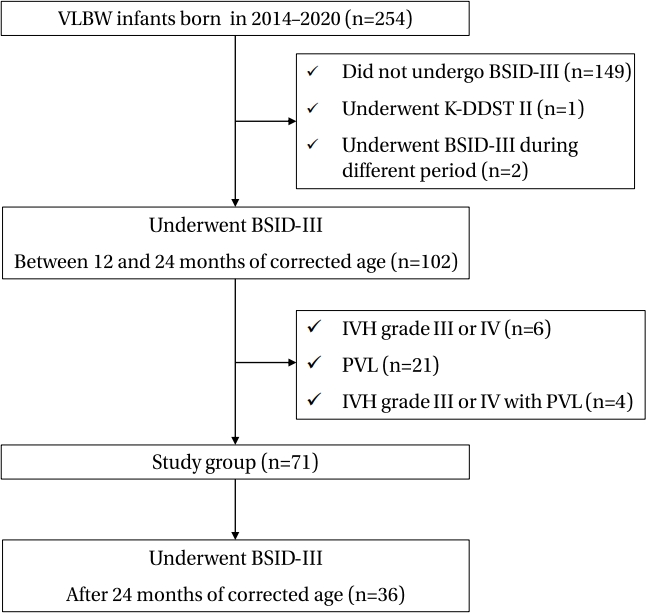
18. Cha JH, Choi N, Kim YJ, Lee HJ, Kim CR, Park HK. Developmental outcome of very-low-birth-weight infants without major brain injuries based on data from the Korean Neonatal Network: a nationwide cohort study. Neonatal Med 2020;27:151–8.

19. Sansavini A, Guarini A, Justice LM, Savini S, Broccoli S, Alessandroni R, et al. Does preterm birth increase a child's risk for language impairment? Early Hum Dev 2010;86:765–72.


20. Kiechl-Kohlendorfer U, Ralser E, Pupp Peglow U, Reiter G, Trawoger R. Adverse neurodevelopmental outcome in preterm infants: risk factor profiles for different gestational ages. Acta Paediatr 2009;98:792–6.


21. Kerstjens JM, Bocca-Tjeertes IF, de Winter AF, Reijneveld SA, Bos AF. Neonatal morbidities and developmental delay in moderately preterm-born children. Pediatrics 2012;130:e265–72.


24. Srinivas Jois R. Neurodevelopmental outcome of late-preterm infants: a pragmatic review. Aust J Gen Pract 2018;47:776–81.


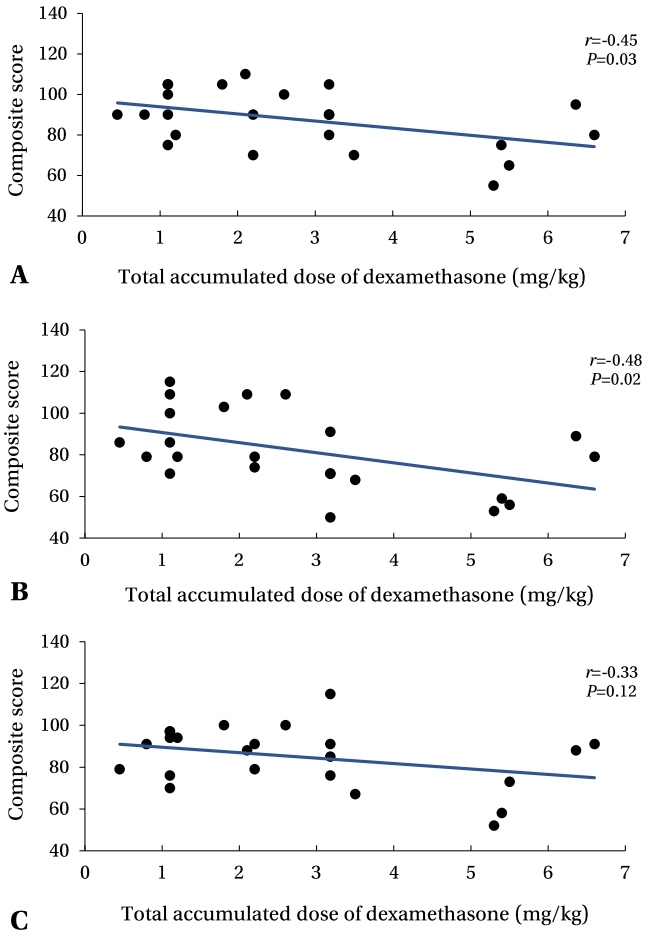
27. Wilson-Costello D, Walsh MC, Langer JC, Guillet R, Laptook AR, Stoll BJ, et al. Impact of postnatal corticosteroid use on neurodevelopment at 18 to 22 months' adjusted age: effects of dose, timing, and risk of bronchopulmonary dysplasia in extremely low birth weight infants. Pediatrics 2009;123:e430–7.


28. Davidovitch M, Kuint J, Lerner-Geva L, Zaslavsky-Paltiel I, Rotem RS, Chodick G, et al. Postnatal steroid therapy is associated with autism spectrum disorder in children and adolescents of very low birth weight infants. Pediatr Res 2020;87:1045–51.


29. Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. An update on the impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk of bronchopulmonary dysplasia. J Pediatr 2014;165:1258–60.


30. Doyle LW, Cheong JL, Ehrenkranz RA, Halliday HL. Late (> 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev 2017;10:CD001145.


31. Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR. Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. N Engl J Med 2000;343:378–84.


32. Maalouf EF, Duggan PJ, Rutherford MA, Counsell SJ, Fletcher AM, Battin M, et al. Magnetic resonance imaging of the brain in a cohort of extremely preterm infants. J Pediatr 1999;135:351–7.


33. Volpe JJ. Cerebral white matter injury of the premature infantmore common than you think. Pediatrics 2003;112(1 Pt 1): 176–80.


34. de Bruine FT, van den Berg-Huysmans AA, Leijser LM, Rijken M, Steggerda SJ, van der Grond J, et al. Clinical implications of MR imaging findings in the white matter in very preterm infants: a 2-year follow-up study. Radiology 2011;261:899–906.


36. Murner-Lavanchy IM, Kidokoro H, Thompson DK, Doyle LW, Cheong J, Hunt RW, et al. Thirteen-year outcomes in very preterm children associated with diffuse excessive high signal intensity on neonatal magnetic resonance imaging. J Pediatr 2019;206:66–71.


37. Rath CP, Desai S, Rao SC, Patole S. Diffuse excessive high signal intensity on term equivalent MRI does not predict disability: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 2021;106:9–16.