 |
 |
- Search
| Neonatal Med > Volume 26(1); 2019 > Article |
|
Abstract
Neonatal hypoxic-ischemic (HI) brain injury is a major cause of neonatal mortality and long-term neurodevelopmental disabilities. Although promising neuroprotective interventions have been studied, the current management of HI brain injury has been limited to supportive measures and induced hypothermia. In addition to engrafting, migrating toward the damage sites and differentiating into multiple lineages, multipotent neural stem/progenitor cells (NSPCs) also provide trophic/immunomodulatory factors and integrate into the host neurons upon implantation into an HI-injured brain. However, NSPC-based therapies have shown poor cell survival and integration, poor differentiation or restricted differentiation into the glial lineages. Furthermore, to achieve full functional recovery following brain injury, the optimization of cell therapy is needed to recapitulate the precise migration of stem cells to the region of interest and the neural rewiring present in the brain microenvironment. Therefore, the efficacy of NSPCs in the treatment of CNS injury is currently insufficient. Human NSPCs (hNSPCs) were isolated from the forebrain of an aborted fetus at 13 weeks of gestation with full parental consent and the approval of the Institutional Review Board of the Yonsei University College of Medicine. Here, to enhance the regenerative ability of hNSPCs in HI brain injury, cells were either pretreated with pharmacological agents or engineered to serve as vehicles for gene delivery. Furthermore, when combined with a poly (glycolic acid)-based synthetic scaffold, hNSPCs provide a more versatile treatment for neonatal HI brain injury. Finally, hNSPCs transfected with zinc-doped ferrite magnetic nanoparticles for controlling both cell migration and differentiation offer a simple and smart tool for cell-based therapies.
저산소 허혈 뇌 손상 신생아의 20%–50%는 신생아기에 사망하며, 중등도 뇌 손상인 경우 생존아의 30%–50%에서, 중증 뇌 손상인 경우 90% 이상에서 중증 신경발달학적 후유증이 동반된다[1,2]. 지난 수십 년 동안 고위험 신생아에 대한 주산기 치료가 획기적으로 발전하였고 새로운 신경보호제 개발을 위한 많은 연구가 진행되었지만, 현재 중등도 이상의 저산소 허혈 뇌증 환아에서 유도 저체온증 치료 시 제한적 신경보호 효과 외에는[3], 신생아 저산소 허혈 뇌손상에 대한 특별한 치료법은 없으며, 주로 일반 대증요법이 시행되고 있다. 따라서 줄기세포를 이용한 세포치료는 특별한 치료법이 없는 신생아 저산소 허혈 뇌 손상에 대한 새로운 신경재생치료법으로 연구되고 있다.
신경줄기세포(neural stem/progenitor cells, NSPCs)는 미분화된 상태로 계속 증식하는 자가갱신(self-renew)을 보이고, 신경계를 이루는 신경세포(neuron), 희소돌기아교세포(oligodendrocyte) 및 별아교세포(astrocyte)로 분화하는 분화의 다능성 을 보이는 세포로 정의하는데[4-7], 한번 손상되면 재생되지 않는 것으로 알려져 있는 인간을 포함한 포유류의 중추신경계에도 NSPC가 존재하고 이 세포는 일생을 통하여 증식ᆞ분화하여 새로운 신경원 및 교세포를 생성한다. 인간 NSPC는 재태 기간 8–20 주 태아 전뇌(forebrain) 혹은 성인의 뇌실하층(subventricular zone) 및 해마 치상회(dentate gyrus)의 과립하층(subgranular layer) 부위 조직을 분리하여 세포증식인자(basic fibroblast growth factor [bFGF] and/or epidermal growth factor [EGF])가 함유된 배지를 사용하여 신경구(neurospheres) 형태로 배양된다[8-11].
NSPC를 퇴행성 신경계질환 모델 동물의 뇌에 이식하면 이식세포는 숙주 신경계 내에서 생착, 생존, 이주하며, 신경계 미세환경 신호 혹은 신경계질환 특이적인 신호에 반응하여 적절한 신경세포로 분화되어 손상된 신경세포를 대체하고 신경회로의 재생을 유도한다. NSPC를 신경원 혹은 신경교세포로 분화 유도한 후 숙주 신경계에 이식하면 손상된 신경세포를 보다 효율적이고 특이적으로 재생할 수 있고, 숙주 신경계에 통합(integration)되어 신경접합(synaptogenesis)을 형성하고 신경전달물질을 분비하여 국소 신경재분포(reinnervation)를 형성하여 신경기능의 호전을 보였다. 또한 이식세포는 신경 손상부위로 특이적으로 이주하거나, 전체 신경축(neuraxis)에 걸쳐 광범위하게 이주하여 신경계질환에서 손상/결함을 보이는 세포, 효소, 신경영양인자, 신경전달물질, 수초(myelin), 세포외기질(extracellular matrix), 세포부착분자(cell adhesion molecules) 등을 제공하였고, 그 외 이식세포는 다양한 신경영양인자(nerve growth factor [NGF], brain-derived neurotrophic factor [BDNF], neurotrophin-3 [NT-3 or NTF3], neurotrophin-4/5 [NT-4/5 or NTF4/5], vascular endothelial growth factor [VEGF], bFGF, glial cell line-derived neurotrophic factor [GDNF] 등), 항염증 싸이토카인/면역제어물질(tumor necrosis factor-α [TNF-α], TL1-A, TNF-related apoptosis-inducing ligand [TRAIL], Fas Ligand [CD95L], prostaglandin E2 [PGE2], nitric oxide [NO], interleukin 1 receptor antagonist [IL-1Ra], interleukin-4 [IL-4], interleukin-10 [IL-10], transforming growth factor-β [TGF-β]), chemokines, chemokine receptors 등을 분비하여 신경재생을 촉진하고, 혈관생성을 유도하였다[12-26]. 그러나 NSPC를 신경계질환 부위에 이식한 경우 일반적으로 숙주 신경계 내에 이식세포의 생착, 생존 및 통합률이 불량하고, 특이 신경세포로의 분화가 잘 안 되거나 주로 신경교세포로 분화하며, 해부학적/기능적으로 정밀한 신경연결망 형성도 이루어지지 않아 현재로는 NSPC의 이식 치료효과가 제한적이다[19,20,25-30].
신생아 저산소성 허혈성 뇌 손상 동물 모델에 인간 NSPC를 이식한 경우, 줄기세포는 뇌 손상 부위에 생착하고, 광범위하게 이주하며, 신경원 및 교세포로 분화하고, 일부는 미분화 상태로 있었으며, 이식세포는 글루타메이트(glutamate), 가바(GABA), 아세틸콜린(acetylcholine) 등의 신경전달물질을 발현하고, 숙주 신경세포와 신경접합 형성함이 확인되었다. 숙주동물 뇌 조직학적 검사상 대조군에 비해 뇌경색증 범위는 유의하게 감소하지는 않았으나, 신경학적 검사상 일부 신경기능의 호전을 보였고, 비정상적인 신경행동 소견이나 뇌종양 형성 등은 보이지 않았다. 이러한 연구 결과를 바탕으로 본 연구자들은 ‘중증 주산기 저산소성 허혈성 뇌 손상 환자에서 동종 인간 NSPC 이식에 의한 세포치료법 확립’이란 연구자 주도 임상시험을 지난 수년에 걸쳐 총 41예의 환자를 대상으로 실시하였고, 인간 NSPC 이식은 안전하고 적용 가능한 세포치료법임을 확인하였다. 본 임상연구는 소규모의 환자를 대상으로 하였고, 이중맹검(double blind) 무작위 대조시험이 아니고, 대상 환아가 초미숙아에서 만삭아까지 다양하며, 뇌 자기공명영상(magnetic resonance imaging, MRI) 검사상 환자마다 저산소성 허혈성 뇌 손상의 해부학적 위치, 정도, 범위 및 신경병리 소견이 다양하고, 세포이식 시 환자의 연령도 다르며, 세포이식 시기도 아급성에서 만성까지 범위가 넓고, 신생아의 뇌는 발달단계에 있어 연령마다 평가 기준이 달라져 세포이식 후 장기간 추적검사가 필요하다. 그러므로 현재는 세포치료의 유효성에 대한 명확히 평가를 할 수 없으나, 일부 환자에서는 세포이식 후 뇌경색증 범위가 유의하게 감소하고 약간의 신경학적 기능 호전이 확인되었다[31].
이상의 결과로 신생아 저산소 허혈 뇌 손상 환아에서 인간 NSPC 이식은 안전하고 적용 가능한 세포치료법임을 제시하였으나, 유효성을 평가하기 위하여 향후 적절한 대상 환아 선택, 이식 시기 결정, 적정 줄기세포 용량 결정, 효과적인 유효성 평가방법 개발, 대규모 환자를 대상으로 이중맹검 무작위 다기관 임상시험 시행 등이 진행되어야 하며, 또한 치료적 유효성이 획기적으로 개선된 기능성 NSPC를 개발하기 위하여 줄기세포 치료 기전 연구와 유전공학, 조직공학 및 나노공학적 융복합 연구를 통한 차세대 세포치료법 개발이 필요하다. 따라서 본 종설에서는 신생아 저산소 허혈 뇌 손상 모델에서 그동안 진행되어 온 융복합 연구를 통한 치료적 유효성이 향상된 기능성 인간 NSPC 기반 세포치료법 개발(neurogenin-2 [NEUROG2] 혹은 interleukin-10 발현 인간 NSPC 이식, TNF-α 전 처치한 인간 NSPC 이식, 생분해성 고분자화합물 비계와 공배양한 인간 NSPC 이식, 자성나노입자 [magnetic nanoparticle, MNP]를 이용한 인간 NSPC 이식) 결과를 약술하였다.
대표적인 proneural basic helix-loop-helix 전사인자인 NEUROG2는 신경계 발달 시 신경세포 생성에 중요한 역할을 하며, NEUROG2 발현 NSPC는 주로 신경세포로 분화한다[32-34]. 또한 NEUROG2 발현 NSPC를 척수손상 모델에 이식한 경우 이식세포의 생존율 증가, 손상 받은 하지의 운동 및 감각기능 호전이 보고되었다[33,34]. 따라서 본 연구자들은 아데노바이러스 벡터를 사용하여 NEUROG2 발현 인간 NSPC (NEUROG2-hNSPCs)와 대조군 green fluorescence protein (GFP) 발현 인간 NSPC (GFP-hNSPCs)를 신생아 저산소 허혈 뇌 손상 모델에 각각 이식하여 치료적 유용성을 비교 분석하였다[35]. 시험관에서 NEUROG2-hNSPCs와 대조군 세포는 각각 약 95%, 39%에서 초기 신경세포의 표지자인 TUJ1 (neuronal class III β-tubulin)을 발현하였고, 특히 NEUROG2-hNSPCs은 TBR1 (cortexspecific neuronal marker), CUX1 (superficial-layer cortical neuronal marker), CTIP2 (deep-layer cortical neuronal marker), vesicular glutamate transporter 1 (vGLUT1, excitatory neuron marker) 발현이 증가하였다. 즉, NEUROG2-hNSPCs가 주로 뇌 피질 상층 및 하층 흥분성 신경세포로 분화하도록 촉진되었다. NEUROG2-hNSPCs 세포를 저산소 허혈 뇌 손상 부위에 이식한 경우 이식세포는 뇌 손상 및 뇌경색증 주변 부위에 잘 생착하고 분포하였으며, 분화 상태는 77%는 TUJ1, 45%는 Nestin (미분화 세포 표지자), 41%는 미분화 세포 혹은 별아교세포의 표지자인 glial fibrillary acidic protein (GFAP), 39%는 역시 미분화 세포 및 별아교세포의 표지자인 STEM123, 5%는 희소돌기아교세포 전구세포의 표지자인 oligodendrocyte transcript factor (OLIG2)를 발현하였고, 반면에 GFP-hNSPCs 세포를 이식한 경우는 대부분의 세포가 Nestin을 발현하였고, 77%는 GFAP, 80%는 STEM123을 발현하였으며, 소수의 세포만이 TUJ1 (18%), OLIG2 (7%)를 발현하였다. 따라서 저산소 허혈 뇌 손상 부위에 이식된 NEUROG2-hNSPCs 세포는 뇌 손상 및 뇌경색증 주변 부위에 잘 생착, 생존, 분포하였으며, 주로 흥분성 신경세포로 분화한 반면, 이식된 GFP-hNSPCs 세포는 대부분 미분화 세포로 존재하였다. 신경학적 검사상 NEUROG2-hNSPCs를 이식한 경우 vehicle을 이식한 대조군과 비교하여 이식 3, 5, 7주에 유의하게 운동-감각 신경기능의 호전을 보인 반면, GFP-hNSPCs를 이식한 경우 이식 5주에서만 유의한 호전을 보였다. 실험동물의 악력 검사(grip strength test)에서는 NEUROG2-hNSPCs를 이식한 경우 vehicle 이식 대조군과 비교하여 이식 5, 7주에 유의하게 앞발의 신경근육기능의 호전을 보인 반면, GFP-hNSPCs를 이식한 경우 대조군과 비교하여 유의한 기능 호전을 관찰할 수 없었다. 따라서 저산소 허혈 뇌 손상 부위에, NEUROG2-hNSPCs를 이식한 경우, GFP-hNSPCs 이식에 비해 더욱 현저한 기능 호전을 관찰할 수 있었다. 저산소 허혈 뇌 손상 부위에 NEUROG2-hNSPCs 이식이 세포사멸에 미치는 영향을 분석하기 위하여 세포 이식 5주 후 TUNEL 분석을 시행하였는데, vehicle 및 GFP-hNSPCs를 이식한 경우와 비교하여 현저하게 세포사멸이 감소하였으나, 세포이식 7주 후에 뇌경색증 범위는 vehicle, GFPhNSPCs 및 NEUROG2-hNSPCs 이식군에서 각 군 간에 차이를 보이지 않았다. 저산소 허혈 뇌 손상 부위에 NEUROG2-hNSPCs 이식이 신경돌기(neurite) 및 축삭돌기(axon)의 신전(extension)에 미치는 영향을 확인하기 위하여 silver 염색 및 axonal tracer인 bitotinylated dextran amine (BDA)를 주사한 후 조직학적 염색을 실시하였는데, NEUROG2-hNSPCs 이식은 대조군들에 비해 뇌 손상 주변 대뇌피질 신경세포의 신경돌기 신전을 유의하게 증가시켰으며, 뇌량(corpus callosum), 뇌 손상 동측 선조체(striatum), 뇌 손상 반대측 경추 제 5번 척수(C5 spinal cord)의 dorsal funiculus 부위에서 신경 축삭돌기의 신전이 크게 향상되었음을 관찰하였다. 따라서 저산소 허혈 뇌 손상에서 NEUROG2-hNSPCs 이식이 대조군들에 비해 대뇌피질 신경세포 신경돌기의 성장과 신전, 손상된 corticostriatal 및 corticospinal tract 축삭돌기의 성장과 신전을 촉진시켜 신경기능적 및 신경해부학적 재생이 유의하게 발생함을 확인하였다. 저산소 허혈 뇌 손상에서 NEUROG2-hNSPCs 이식의 신경재생/신경보호 작용 기전을 규명하기 위하여 세포에서 발현/분비되는 신경영양/보호인자를 측정하였는데, NEUROG2-hNSPCs 세포는 GFP-hNSPCs 세포에 비해 BDNF, NGF, fibroblast growth factor 9 (FGF9), ciliary neurotrophic factor (CNTF), thrombospondin 1/2 (THBS 1/2)의 발현/분비가 크게 증가되었으며, 이러한 인자들이 실제로 신경세포 사멸을 감소시키고, 신경세포 돌기의 신전을 향상시킴을 확인하였다. 결론적으로 신생아 저산소 허혈 뇌 손상 부위에 대조군 인간 NSPC인 GFP-hNSPCs 보다는 NEUROG2-hNSPCs 세포를 이식할 경우, 이식세포가 주로 신경세포로 분화하고 다양한 신경영양인자들을 활발히 분비하여 뇌 손상 부위의 병리적 미세환경을 변화시킴에 따라 신경세포의 생존 및 신경가소성이 현저히 증가하고 신경기능 회복이 크게 촉진되어, 향후 신생아 저산소 허혈 뇌 손상 환아에서 인간 NSPC 기반 세포-유전자 치료 적용 시 치료적 유효성을 향상시킬 것으로 기대된다.
저산소 허혈 뇌 손상 초기 단계(initial phase)에서는 세포 및 조직에 에너지가 고갈되어(primary energy failure) 즉각적으로 신경세포가 사망하고(primary neuronal death), 적어도 몇 시간 또는 수일 이내로 흥분독성(excitotoxicity), 세포사멸(apoptosis) 및 염증반응 등으로 인하여 이차 신경세포 사망(delayed neuronal death)이 발생한다. 손상된 신경세포 및 희소돌기아교전구세포(oligodendroglial precursors)는 danger/damage-associated molecular patterns, ATP, 흥분독소(excitotoxins) 및 기타 alarm signals를 분비하여 면역작동세포(immune effector cells; microglia, macrophages, astroglia, mast cells)의 pattern recognition receptors/cytokine receptors를 통하여 내재면역반응(innate immune response)을 촉발한다[36,37]. 자극된 면역작동세포는 proinflammatory cytokines, chemokines, reactive oxygen/nitrogen species, excitotoxins, histamines, prostaglandins, leukotrienes, death receptor agonists (TNF, FasL, RANKL, TRAIL, TWEAK) 등을 분비하여 신경세포, 희소돌기아교전구세포, 혈관내피세포 등을 손상시키고, 혈관-뇌 장벽(blood-brain barrier) 투과성을 증가시켜 말초면역세포(neutrophils, mast cells, macrophages, natural killer cells, T cells, myeloid cells 등)들이 뇌 손상 부위로 이주하는 과정을 촉진시킨다. 따라서 저산소 허혈 뇌 손상 시 중추 및 말초 면역세포들이 작동하여 내재 및 적응면역반응(adaptive immune response)에 의한 염증(inflammation)이 발생하며, 이러한 염증반응은 뇌 손상 발생 이전, 도중 및 이후에도 발생하고 적어도 수주간 지속되어 직접적인 뇌세포 손상을 일으키고, 뇌 발달 장애를 유발한다.
최근의 연구에 의하면 신생아 저산소 허혈 뇌 손상 시 초기 염증반응을 감소시키는 다양한 치료제(corticosteroids, minocycline, melatonin, erythropoietin, cyclooxygenase inhibitors, pifithrin-μ, cromolyn, innate defence regulatory peptide, N-acetylcysteine, TNF receptor blockade, IL-1 receptor antagonist, Tat-NEMO-binding domain, simvastatin, histone deacetylase inhibitor)를 사용할 경우 신경보호 효과가 있음이 보고되었는데[36], NSPC를 이식할 경우에도 항염증/면역조절 작용이 있으며[17,38,39], 본 연구자들도 인간 NSPC에서 항염증인자(TGF-β1, IL-4, IL-13, CXCL1, CD47, CD200)들이 발현되며, 특히 알츠하이머병 모델에서 TGF-β1, CD47, CD200 등의 작용에 의하여 활성미세아교세포에 대한 항염증 효과가 있음을 확인하였다[40].
IL-10은 대표적인 항염증 싸이토카인인데, 내재면역세포(dendritic cells, macrophage, natural killer cells, eosinophil, neutrophil), 적응면역세포(T helper 1 [TH1], TH2, TH17, regulatory T cell [Tregs], B cells) 및 신경세포(microglia, astrocyte, neuron) 등에서 염증반응의 negative feedback으로 염증촉진 싸이토카인(proinflammatory cytokines; TNF-α, IL-1β, IL-6, IL-8, IL-12, IL-23, granulocytecolony stimulating factor [G-CSF], granulocyte-macrophage colonystimulating factor [GM-CSF])의 분비를 억제시키고, 내재면역세포로부터 항염증인자인 IL-1Ra 및 soluble TNF receptors (sTNFRs)의 발현을 증가시키며, CD4+ T 세포의 증식과 싸이토카인 합성을 억제하여 TH1 세포에서 IL-2 및 interferon-γ, TH2 세포에서 IL-4와 IL-5 생성을 감소시킨다[41]. 또한 IL-10은 monocytes/macrophages의 항원 발현(antigen presentation)을 억제하여 세포 표면에서 major histocompatibility complex class II (MHC II) 발현을 감소시키며, 세포 분극(cell polarization)을 촉진시켜 macrophages는 IL-10 발현 항염증형 macrophages인 M2-like macrophages로, T 세포는 TH1/TH17 세포보다는 TH2 세포로 그리고 Treg 세포로 분화를 유도하고, CD28 인산화를 통하여 면역성 결여(anergy) 상태를 유발한다. IL-10은 신경보호 작용도 있는데 microglia로부터 염증촉진인자 분비를 감소시켜 별아교세포 활성화를 억제하고, nuclear factor κB (NF-κB) 활성 감소를 통해 별아교세포의 excitatory amino acid transporter-2 (EAAT2) 발현을 증가시켜 synaptic cleft에서 glutamate 축적을 감소시키며, 일부 IL-10 receptor를 발현하는 신경세포에서는 PI3K-AKT pathway를 통하여 세포생존 관련 유전자 발현은 증가시키고 세포 내 칼슘 농도를 정상화시켜 glutamate에 의한 excitotoxicity 및 저산소 허혈 손상으로부터 신경세포를 보호한다. 또한 IL-10은 항세포사멸인자인 Bcl-2/Bcl-xl 발현은 증가시키고, caspase-3 발현은 감소시켜 글루타메이트-유도 세포사멸을 억제하여 신경보호를 유발한다[41]. 동물모델을 이용한 연구에 의하면 IL-10 과발현 형질전환 마우스에 뇌졸중을 유발한 경우 뇌경색증 범위가 감소하였고[42], 아데노바이러스 벡터를 이용하여 IL-10 유전자 치료를 시행한 경우 역시 뇌경색증 범위가 감소하였으며[43], IL-10 발현이 부족한 뇌졸중 마우스는 뇌경색증 범위가 커졌으며[44], 실제 혈청 IL-10 수준이 낮은 뇌졸중 환자는 나쁜 경과를 보였다[45]. 이상의 연구 결과로 IL-10은 체내에서 분비되는 강력한 항염증/면역조절인자이므로 본 연구자들은 신생아 저산소 허혈 뇌 손상 모델에서 인간 NSPC 이식 치료효과를 향상시키기 위하여 인간 NSPC의 항염증/면역조절능이 크게 향상된 IL-10 과발현 인간 NSPC를 이용하여 줄기세포 기반 세포-유전자 치료를 시도하였다.
Lentivirus 벡터를 이용하여 GFP-hNSPCs와 human IL-10 및 GFP 발현 hNSPCs (IL-10-hNSPCs)를 제작한 다음, 시험관 내에서 세포 특성을 분석하였는데, IL-10-hNSPCs는 IL-10 단백질을 87.4±5.72 ng/mL (1×106 cells/2 mL of media/72 hours) 농도로 잘 발현하였고, 반면 GFP-hNSPCs 세포는 IL-10을 발현하지 않았으며, 5-ethynyl-2'-deoxyuridine (EdU)를 이용한 세포증식 분석실험에서는 GFPhNSPCs보다는 IL-10-hNSPCs의 세포증식이 증가함을 보였다. 세포분화 실험에서는 GFP-hNSPCs와 비교하여 IL-10-hNSPCs는 TUJ1은 많이 발현하였고, 반면 Nestin, GFAP 및 OLIG2 발현은 감소하여 IL-10-hNSPCs은 분화조건 아래에서 신경교세포(별아교세포, 희소돌기아교세포)보다는 신경세포로 분화가 촉진되었다. CD1 mouse를 이용하여 생후 7일에 저산소 허혈 뇌 손상을 유발하고 3일 후 IL-10-hNSPCs, GFP-hNSPCs을 각각 뇌 손상 부위에 직접 이식하고 2주 및 4주 후에 분석한 결과, IL-10-hNSPCs은 뇌경색증 접경부위, 뇌백질 부위 등에 광범위하게 생착 및 분포함을 보이고 human IL-10 단백질을 발현하였으며, 45.5%±1.85%의 세포는 TUJ1을 발현하여 GFP-hNSPCs 보다는 약 27.4%에서 더 많이 발현하였고, 그 외 Nestin, GFAP, OLIG2 등을 발현하여 저산소 허혈 뇌 손상 부위에 이식된 IL-10-hNSPCs은 GFP-hNSPCs과 비교하여 더 많은 세포가 신경원세포로 분화하고, 일부는 신경교세포로 분화하였으며, 다수는 미분화 상태를 보였다. 저산소 허혈 뇌 손상 부위에 IL-10-hNSPCs, GFP-hNSPCs, vehicle을 이식하고 1, 2, 3, 4주일 후 신경학적 기능검사를 실시하였는데, IL-10-hNSPCs 이식군은 대조군 및 GFP-hNSPCs 이식군에 비해 전 기간에 걸쳐 신경학적 기능 향상을 보였고, GFP-hNSPCs 이식군은 대조군에 비해 신경학적 기능은 향상을 보였으나 유의한 차이는 보이지 않았다. 앞발의 운동기능을 평가하는 cylinder test에서는 IL-10-hNSPCs 이식군은 대조군에 비해 이식 3, 4주 후 운동기능의 향상을 보였고, GFP-hNSPCs 이식군과 비교하여서도 이식 3주 후 기능의 향상을 보인 반면, GFP-hNSPCs 이식군은 대조군에 비교하여 유의한 차이를 보이지 않았다. 또한 IL-10-hNSPCs 이식군은 세포이식 4주 후 대조군에 비해 약 20%의 뇌경색증 범위의 감소를 보인 반면, GFP-hNSPCs 이식군은 대조군에 비해 뇌경색증 범위의 유의한 차이는 보이지 않았다. 뇌경색증 주변 부위에 세포사멸의 정도를 평가하는 TUNEL 검사에서도, IL-10-hNSPCs 이식군은 대조군 및 GFPhNSPCs 이식군에 비해 크게 세포사멸 정도가 감소하였다.
IL-10-hNSPCs가 염증반응에 미치는 영향을 평가하기 위하여 먼저 시험관 내에서 BV2 세포(mouse microglial cell line)에 지질다당질(lipopolysaccharide, LPS)을 처치하여 활성화 시켰는데, 염증촉진 싸이토카인인 TNF-α, IL-1β, IL-6, inducible nitric oxide synthase (iNOS) 발현 정도가 모두 크게 상승함을 보였다. 여기에 GFPhNSPCs 세포의 conditioned media (GFP-hNSPCs-CM)를 첨가하면 대조군에 비해 IL-6, iNOS 발현 정도가 유의하게 감소하나, IL-10-hNSPCs-CM을 첨가하면 TNF-α, IL-1β, IL-6, iNOS 발현 정도가 모두 유의하게 감소하고, GFP-hNSPCs-CM 군과 비교하여도 TNF-α, IL-6의 발현 정도가 유의하게 감소하였다. 이러한 결과는 RAW264.7 세포(murine macrophage cell line)를 이용하여 다시 확인하였는데, GFP-hNSPCs-CM을 첨가하면 TNF-α, IL-6 발현이 대조군에 비해 감소하였고, IL-10-hNSPCs-CM을 첨가하면 TNF-α, IL-1β, IL-6 발현이 대조군에 비해 모두 유의하게 감소하였으며, GFP-hNSPCs-CM 군과 비교하여도 TNF-α, IL-6 발현이 유의하게 감소하였다. 따라서 인간 NSPC는 활성 microglia 및 macrophage로부터 염증촉진 싸이토카인 발현을 감소시키나, IL-10-hNSPCs는 이러한 항염증 효과가 훨씬 강력하다. 또한 IL-10-hNSPCs는 BV2 세포의 증식에도 영향을 미쳤는데, IL-10-hNSPCs에서 분비되는 인자들뿐만 아니라 막간 단백질(transmembrane proteins)도 BV2 세포의 증식을 억제하였다.
Microglia는 뇌 속에 존재하는 면역세포로, 중추신경계의 발달, 유지 및 재생에 중요한 역할을 담당하며[46], microglia 활성 변화는 편두통, 뇌졸중, 치매, 사고성 뇌 손상, 뇌전증 및 파킨슨씨병과 같은 질환 발생과 연관이 있다[47-49]. Microglia/macrophages는 중추신경계의 재생과 복구에 중요하나 양날의 칼과 같은 역할을 한다[50]. 활성화된 microglia/macrophages는 손상된 세포 잔해를 제거하고 염증반응을 감소시키며 영양인자 등을 분비하여 신경재생/복구를 촉진시키나, 신경재생을 방해하고 신경조직 손상 범위를 확대시키기도 한다[50-55]. 세포 분극적 분류에 의하면 classically activated microglia/macrophages (M1 type)는 염증촉진인자들을 분비하여 신경 손상을 유발하며, alternatively activated microglia/macrophages (M2 type)는 대식작용에 의해 손상된 세포 잔해를 제거하고 신경보호 및 영양인자 등을 분비하여 신경재생/복구를 촉진한다[56,57]. 따라서 본 연구에서는 마우스의 대퇴골 및 경골에서 macrophages를 분리 배양하여 세포 분극의 변화를 flow cytometry 방법으로 측정하였는데, macrophages에 대조군-CM, GFP-hNSPCs-CM, IL-10-hNSPCs-CM을 각각 처리하고 M1 형 macrophages (MHC class II+ & CD206-)의 비율을 분석한 결과, IL-10-hNSPCs-CM 처리군은 대조군 및 GFPhNSPCs-CM 처리군과 비교하여 유의하게 M1 형이 감소하였고, GFP-hNSPCs-CM 처리군도 대조군보다는 M1형이 감소함을 보였다. 반면 M2형 macrophages (MHC class II- & CD206+)의 비율은 IL-10-hNSPCs-CM 처리군은 대조군 및 GFP-hNSPCs-CM 처리군과 비교하여 유의하게 증가하였고, GFP-hNSPCs-CM 처리군도 대조군보다는 M2형이 증가함을 보였다. 즉, 이상의 연구 결과로 IL-10-hNSPCs은 활성화된 microglia/macrophages에서 염증촉진인자들의 발현과 microglia의 세포증식을 감소시키고, 골수 유래 macrophages를 M2형으로 분극 유도함을 확인하였다.
저산소 허혈 뇌 손상 시 초기 수 시간에서 수 일 동안은 신경독성 작용이 있는 염증촉진인자들이 과 발현되어 세포 및 조직 손상을 유발하고, 수 일 후에는 항염증 싸이토카인들이 발현되며, 그 후 수 일에서 수 주 동안은 손상된 세포 잔해가 제거되고 신경영양인자들이 분비되어 신경복구가 이루어진다[36]. 따라서 본 연구에서는 저산소 허혈 뇌 손상 유발 3일 후 IL-10-hNSPCs를 뇌 손상 부위에 이식하고 1주일 후 뇌 조직을 추출하여 초기 염증반응에 미치는 효과를 분석하였는데, IL-10-hNSPCs 이식군은 대조군에 비해 IL-1β, IL-6, iNOS의 mRNA 발현 정도가 모두 감소함을 보였고, GFP-hNSPCs 이식군에 비해서도 IL-6가 유의하게 감소하였고, 특히 IL-10-hNSPCs 이식군은 GFP-hNSPCs 이식군과 대조군에 비해 TNF-α 및 IL-β 단백질 발현 정도가 유의하게 감소하였다. 또한 뇌 손상 조직에서 CD11b 항체가 접합되어 있는 magnetic beads를 사용하여 CD11b+ 세포들을 분리한 다음, M1 형 및 M2 형 microglia/macrophages 표지자 유전자들을 real-time PCR로 분석하였는데, TNF-α (M1 형 표지자)는 IL-10-hNSPCs 이식군에서 대조군과 비교하여 차이가 없었고, GFP-hNSPCs 이식군에 비해서는 증가함을 보였으나, arginase 1, IL-10 및 CD206 (M2 형 표지자)은 IL-10-hNSPCs 이식군에서 대조군과 비교하여 모두 유의하게 크게 증가하였고, GFP-hNSPCs 이식군은 arginase 1이 크게 증가함을 보였다. 이러한 결과는 시험관에서 BV2 세포를 이용한 실험에서 동일한 결과를 확인하였다. 따라서 저산소 허혈 뇌 손상 부위에 IL-10-hNSPCs을 이식한 초기에는 손상된 조직에서 염증촉진 싸이토카인들의 발현은 크게 감소하며, M1형 microglia/macrophages는 불활성화되고 M2형은 활성화되었다. 저산소 허혈 뇌 손상 부위에 IL-10-hNSPCs를 이식하고 후기에 염증반응에 미치는 영향을 분석하기 위하여 이식 4주 후 뇌 조직에서 Iba1 항체를 이용하여 면역조직화학적 염색을 시행하였는데, 뇌 손상 부위와 주변 부위에서 IL-10-hNSPCs 및 GFP-hNSPCs 이식군은 대조군에 비해 Iba1+ (microglia/macrophages 표지자) 세포 수가 크게 증가하였으나, CD68+ (activated microglia/macrophages 표지자) 세포 수는 IL-10-hNSPCs 이식군이 GFP-hNSPCs 이식군과 비교하여 뇌 손상 동측 및 반대 측에서 모두 유의하게 감소하였다.
IL-10-hNSPCs의 항염증/면역조절 작용 외에 신경보호 및 신생혈관생성에 미치는 영향을 분석하였는데, 시험관 내에서 human neuroblastoma SH-SY5Y 세포를 신경세포로 분화시켜 정상 산소 농도 및 무산소 농도 조건으로 fibroblast, GFP-hNSPCs 및 IL-10-hNSPCs와 공동 배양 후 세포독성을 평가한 결과, IL-10-hNSPCs와 공동 배양한 경우 가장 세포독성이 감소하였으며, IL-10-hNSPCs는 GFP-hNSPCs와 비교하여 높은 농도의 NGF, NT3, NT4 및 BDNF를 발현하였다. 또한 IL-10-hNSPCs는 GFP-hNSPCs와 비교하여 높은 농도의 matrix metalloproteinase 1 (MMP1), matrix metalloproteinase 2 (MMP2) 및 plasminogen activator inhibitor-1 (PAI-1)을 발현하여 신생혈관 생성을 촉진하였다. 이상의 연구 결과로 신생아 저산소 허혈 뇌 손상 모델에 IL-10 발현 인간 NSPC 이식은 항염증/면역조절, 신경보호 및 혈관생성 작용 등 다양한 기전으로 신경재생 및 복구를 유발하여 향후 신생아 저산소 허혈 뇌 손상 환아에서 인간 NSPC 기반 세포-유전자 치료 적용 시 치료적 유효성을 크게 향상시킬 것으로 기대된다.
저산소 허혈 뇌 손상에 NSPC를 이식할 경우 다양한 기전에 의해 기능적 및 조직학적으로 호전을 보임에도 불구하고, 저산소 허혈 미세환경에서 이식세포의 생착 및 생존이 불량하고 세포사멸이 증가하여 현재로는 NSPC의 이식 치료효과가 제한적이다[30,58,59]. 따라서 세포치료 유효성을 증가시키기 위해서는 저산소 허혈 뇌 손상 환경에서 이식세포의 사멸을 감소시키는 것이 중요한데, 바이러스 벡터를 사용하여 세포생존관련 유전자 혹은 분비인자를 과 발현시킨 줄기세포를 이식한 경우 치료효과가 향상됨이 보고되었으나[60-62], 임상적용 시 유전공학기술의 안전성 및 외부 유전자 발현조절기술 측면에서 문제가 될 수 있어, 보다 안전하고 간편한 방법으로 이식세포의 생존을 증가시키는 기술 개발이 필요하다. 이러한 유전공학적 형질 전환 대신 최근 NSPC를 약제로 전 처치하고 뇌 이식한 경우 세포 생존율이 증가하고 신경기능이 호전됨이 보고되었는데[58,63,64], 본 연구자들은 특히 TNF-α에 주목하였다.
TNF-α는 대표적인 염증촉진 싸이토카인인데, 뇌 손상 시 활성화된 microglia/macrophages에서 수 분 이내로 발현되기 시작하여 수일간 발현이 지속되며, 다양한 퇴행성 신경계질환(헌팅톤병, 알츠하이머병, 파킨슨병, 근위축성 측삭경화증, 뇌졸중 등)에서 염증반응의 핵심적인 역할을 담당한다[59,65]. 한편 TNF-α는 뇌졸중에서 신경보호 작용이 보고되었고[66], 내인성 NSPC의 증식, 분화 및 생존에도 영향을 미쳐 뇌졸중, 퇴행성 신경계질환, 신경염증질환 후 조직 리모델링 및 복구에 중요한 조절 역할을 함이 보고되었다[67-69]. TNF-α는 NSPC의 TNF-α receptor에 작용해 mitogen-activated protein kinase (MAPK)를 활성화시키는데[70], 본 연구에서는 인간 NSPC에 TNF-α를 전 처치하여 세포 내 신호전달기전을 규명하고, 저산소 허혈 뇌 손상 부위에 이식할 경우 이식세포의 생존율 및 세포치료의 유효성을 미치는 영향을 조사하였다.
시험관 내의 산소-포도당 차단(oxygen glucose deprivation, OGD) 조건 하에서 인간 NSPC의 생존율은 크게 감소하는데, 다양한 농도의 TNF-α를 전 처치한 결과 세포 생존율이 크게 증가하고 세포독성은 감소하였으며, 20 ng/mL의 TNF-α 농도에서 세포사멸이 가장 적게 발생하였다[71]. 저산소 허혈 뇌 손상을 유도하고 3일 후 TNF-α (20 ng/mL) 전 처치한 인간 NSPC (TNF-α-hNSPCs)와 전 처치하지 않은 대조군 세포(hNSPCs)를 뇌 손상 부위에 이식하고 매주 간격으로 4주 동안 뇌 손상 부위에 이식세포의 생착 및 생존을 bioluminescence image (BLI) 및 면역조직화학적 방법으로 분석하였는데, TNF-α-hNSPCs를 이식한 경우 대조군에 비해 세포 생착 및 생존이 2–3배 이상 크게 증가함을 보였다. OGD 조건 하에서 TNF-α 전 처치가 인간 NSPC의 생존에 미치는 기전을 분석하였는데, 세포 내 NF-κB 및 p38 MAPK 신호기전이 활성화되었고, 각각의 신호전달체계의 inhibitors를 사용하여 분석한 결과, 특히 NF-κB pathway에 의해 세포 생존이 증가함을 보였다[71]. TNF-α 전 처치가 인간 NSPC의 유전자 발현에 미치는 영향을 분석하기 위하여 RNA-seq 분석 방법을 사용하였는데, TNF-α-hNSPCs는 대조군에 비해 항세포사멸 유전자인 cIAP2 와 항산화 유전자인 SOD2 발현이 증가함을 보였고, TNF-α 유도 cIAP2 단백질의 과발현은 p38 MAPK 보다는 NF-κB 신호전달체계에 의해 유도됨을 확인하였으며, 저산소 허혈 뇌 손상 부위에 cIAP2 유전자 발현을 억제한 인간 NSPC (lentivirus-shcIAP2-trasnfected hNSPCs)를 TNF-α로 전 처치하여 이식한 경우 이식세포의 생착 및 생존율이 50% 이하로 감소함을 확인하였다. 저산소 허혈 뇌 손상 부위에 TNF-α-hNSPCs 이식은 이식세포의 생존율을 증가시키는데, 실제 이식 후 9주까지 매주 신경기능에 미치는 영향을 rotarod test, cylinder test, neurological severity scoring 검사 등으로 분석한 결과, TNF-α-hNSPCs 이식은 대조군과 비교하여 운동 및 감각 신경기능을 크게 개선함을 확인하였고, 세포이식 9주 후 뇌경색증 범위를 평가한 결과 대조군과 비교하여 약 14% 감소하였으며, TUNEL 검사상 세포사멸 정도는 약 4배 감소하였고, caspase-3 발현 정도도 감소함을 보였다. 이상의 결과로 저산소 허혈 뇌 손상 부위로 TNF-α-hNSPCs 이식은 대조군과 비교하여 유의한 신경보호 효과를 보였다. 또한 TNF-α-hNSPCs 이식이 microglia에 미치는 영향을 분석하였는데, 면역조직화학검사상 저산소 허혈 뇌 손상 부위에 활성 microglia의 표지자인 CD68+ 염색 정도가 TNF-α-hNSPCs 이식군에서 대조군에 비해 유의하게 감소하였고, 염증촉진 싸이토카인 TNF-α, IL-1β 및 IL-6 발현도 대조군에 비해 감소함을 보였다. TNF-α-hNSPCs의 항염증작용을 조사하기 위하여 시험관에서 LPSactivated BV2 cell line에 TNF-α-hNSPCs-CM (conditioned media)을 첨가한 경우 TNF-α, IL-1β, IL-6 및 iNOS 발현이 감소하였고, TNF-α 전 처치가 인간 NSPC에서 염증관련 유전자의 발현에 미치는 영향을 알기 위하여 RNA-seq 분석을 시행한 결과, fractalkine/CX3CL1 발현이 대조군에 비해 약 25배 증가함을 확인하였다. Fractalkine/CX3CL1의 발현은 다른 세포들에서 TNF-α에 의해 조절됨이 보고되었고[72,73], microglia 활성을 억제하여 염증촉진 싸이토카인 발현을 감소시킨다[74,75]. 본 연구에서도 TNF-α-hNSPCs-CM에서 anti-CX3CL antibody를 이용하여 CXCL1을 immunodeletion한 후 활성화된 microglia와 공동 배양하면, 대조군과 비교하여 TNF-α, IL-1β 및 IL-6 발현이 감소하여 TNF-α를 전 처치할 경우 fractalkine/CX3CL1 발현이 증가하여 염증촉진 싸이토카인의 발현을 감소시킴을 확인하였고, SH-SY5Y 세포 유래 신경세포와 공동배양 시에도 TNF-α-hNSPCs-CM 유래 fractalkine/CX3CL1이 microglia로부터 염증촉진 싸이토카인의 발현을 감소시켜서 신경세포 사멸을 감소시킴을 확인하였다.
이상의 연구 결과로 인간 NSPC에 TNF-α를 전 처치할 경우 NF-κB 신호전달을 통하여 cIAP2 유전자 발현이 증가하여 시험관 내 산소-포도당 차단 조건 및 생체 내 저산소 허혈 뇌 손상 부위에서 세포 생존율이 증가하고, 역시 NF-κB 신호전달을 통하여 CXCL1 유전자 발현이 증가하여 항염증작용에 의한 신경보호 효과를 보이고, TNF-α-hNSPCs를 저산소 허혈 뇌 손상 부위에 이식한 경우 대조군과 비교하여 유의하게 뇌경색증 범위가 감소하고 신경기능이 개선됨을 보였다. 향후 신생아 저산소 허혈 뇌 손상 환아에서 인간 NSPC 기반 세포치료 적용 시 간단하고 안전한 방법으로 치료적 유효성을 크게 향상시킬 것으로 기대된다.
신생아 저산소 허혈 뇌 손상이 중증일 경우 광범위한 조직 손상으로 인하여 뇌연화증(encephalomalacia) 혹은 대뇌 반구(cerebral hemisphere)에 큰 낭종성 와동(cystic cavity)을 형성한다. 뇌 손상 질환에서 NSPC는 높은 신경재생/복구 능력을 보이지만, 이러한 중증 뇌 손상 부위에 이식된 NSPC는 신경재생을 유도할 내인성 신경조직 및 성형(template)이 부족하고 혈액공급도 일어나지 않아 신경재생/복구가 제한되며, 이식세포의 생착 및 생존도 감소한다.
최근에 조직공학적 방법으로 신경조직 재생을 유도하는 다양한 생체재료(biomaterials)가 개발되었는데, 3차원 구조와 거미줄 같은 섬유질 형태 및 특이 미세구조의 세포외기질 특성 등을 보여 치료적 유용성을 보이는 약물, 단백질 혹은 줄기세포를 중추신경계 특정 부위에 전달이 가능하며 손상된 신경조직의 재생/복구, 신경연결망의 복구를 촉진함이 보고되었다[27,76-78]. 본 연구자들은 신생 마우스의 소뇌에서 추출된 불사화(immortalized)된 NSPC와 이식세포의 조직화와 성장, 이주 및 분화를 유도하는 성형인 생분해성 고분자화합물 비계를 공동 배양한 후, NSPC-비계 복합체를 신생아 저산소 허혈 뇌 손상 부위에 이식하였는데, 이식세포가 잘 생착하고, 뇌 손상 용적이 감소하였고, 혈관생성, 신경세포로의 분화 및 신경돌기 신전이 촉진되었으며, 신경연결망 형성이 유도됨을 관찰하였다[15]. 이러한 결과는 향후 중증 저산소 허혈 뇌 손상 시 고분자화합물 비계와 NSPC 복합체를 이용하여 신경조직의 재생 및 복구가 가능함을 제시하였다. 따라서 본 연구자들은 안전하고 임상적용이 가능한 생분해성 합성비계의 일종인 poly (glycolic acid) (PGA)에 인간 태아 뇌 유래 일차 NSPC를 공동 배양하여 줄기세포-생체재료 비계 복합체를 신생아 중증 저산소 허혈 뇌 손상 부위에 이식하여 NSPC-생체재료 비계 기반 조직공학적 신경조직 재생 치료 가능성을 연구하였다.
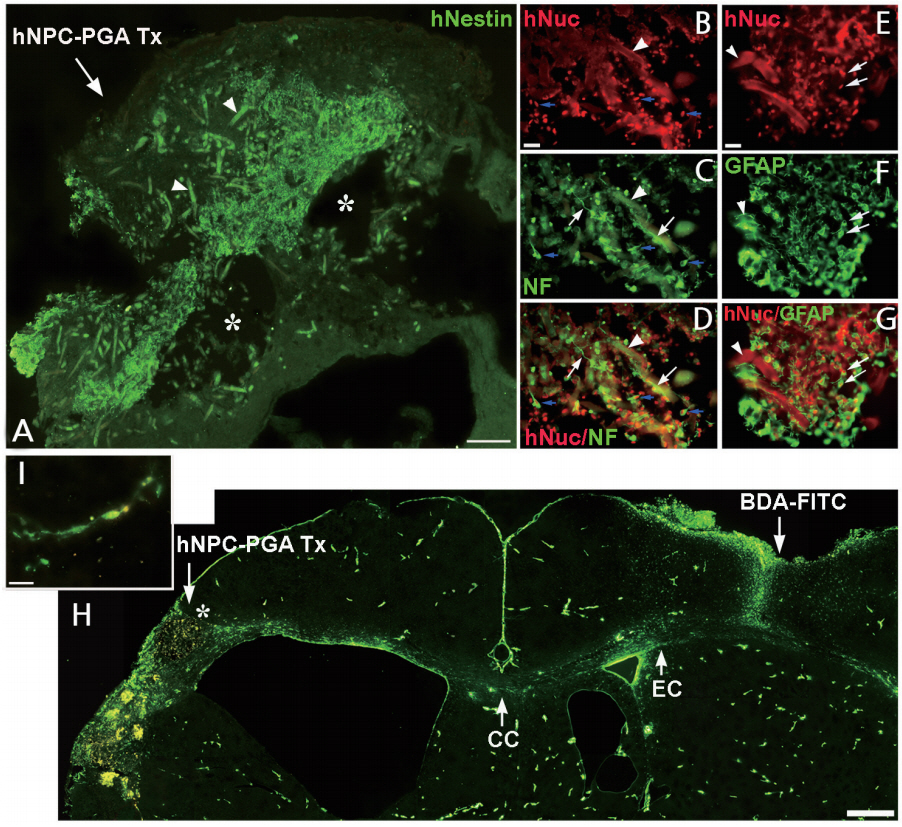
PGA 비계에 인간 NSPC를 공동 배양한 결과, 세포는 비계에 잘 생착, 이주 및 성장하고 신경원 및 교세포로 분화하였으며, 신경세포로 분화한 경우 신경돌기를 길게 신장하고 비계를 둘러싸며 비계 간에 연결을 촉진하였다. 시험관에서 PGA 비계는 세포독성을 보이지 않고 인간 NSPC의 성장과 분화를 촉진시켜 PGA 비계-인간 NSPC 복합체(hNSPCs-PGA complexes)는 생체 내 이식에 적합함을 보였다. 저산소 허혈 뇌 손상 7일 후 hNSPCs-PGA를 우측 대뇌반구(cerebral hemisphere)에 형성된 큰 뇌경색증 와동 부위에 이식하였는데, hNSPCs-PGA는 뇌 손상 부위에 잘 생착하고 뇌경색증 범위를 감소시켰으며, 숙주 뇌 조직에 통합(integration)됨을 보였다. 면역조직화학검사상 뇌 손상 부위에는 복합체의 이식으로 새로운 조직으로 채워져 있고, 이식세포의 약 20%는 TUJ1+, 약 35%는 GFAP+, 80% 이상은 Nestin+임을 보여, 일부 이식세포는 신경세포로 분화하여 신경돌기를 길게 신전하였으나, 대부분의 세포는 미분화 상태에 있었고, 이식 2개월 후에는 이식세포 존재를 확인하기 어려웠다. 따라서 이식세포는 면역억제제를 사용하여도 중증 저산소 허혈 뇌 손상 부위에 이종이식(xenotransplantation)한 경우 장기간 생존이 어렵고, hNSPCs-PGA 이식은 뇌 손상 부위를 감소시키며 새로운 조직을 형성함에도 불구하고 이식세포가 분화하여 손상된 신경세포를 재생/대체함에서는 한계를 보였다. 뇌 손상 부위에 hNSPCs-PGA, PGA, hNSPCs 및 vehicle (대조군) 이식 8주 후 조직학적 검사상 hNSPCs-PGA 이식한 경우만 유의하게 뇌경색증 범위의 감소함을 보였고, 뇌 손상 부위에 새롭게 형성된 조직에서는 신생혈관형성이 크게 촉진되고 숙주 혹은 이식세포에서 유래된 신경세포의 복잡한 신경돌기들이 뇌 손상 주변 숙주 뇌 조직과 연결되어 보였다. 따라서 hNSPCs-PGA 이식으로 새롭게 생성된 조직은 숙주 뇌 조직에 통합되었고, 혈관생성인자와 신경돌기 및 축삭돌기 신전(neurite extension and axonal outgrowth) 유발인자들이 함께 작용하여 숙주 뇌 조직과 밀접히 연관됨을 확인하였다. 실제로 뇌 손상 부위로 이식된 hNSPCs-PGA가 숙주 뇌의 특정 부위와 신경연결을 이루는 것을 확인하기 위하여 hNSPCs-PGA 이식 10주 후에 신경축삭돌기 추적자(axonal tract tracer)인 biotinylated dextran amine conjugated with fluorescein (BDA-FITC)을 뇌 손상 반대쪽 정상 대뇌피질에 주사하고 2주 이상 경과한 후 뇌 조직을 분석한 결과, fluorescein-착색된 축삭돌기들이 정상 대뇌 반구의 피질, 대뇌 백질의 바깥섬유막(external capsule), 뇌량 및 hNSPCs-PGA가 이식된 반대쪽 대뇌 반구의 뇌 손상 부위로 연결됨을 보였다. 특히 뇌 손상 주변 대뇌피질 부위에서 신경세포와 유사한 형태를 가진 세포의 세포체(cell body)와 세포돌기(cellular processes)가 BDA-FITC로 염색됨을 확인하였다. 그러나 PGA, hNSPCs 및 vehicle만 이식한 경우는 이러한 신경축삭돌기의 연결형태를 확인할 수 없었다. 이상의 결과로 저산소 허혈 뇌 손상 부위에 hNSPCs-PGA 복합체 이식은 일부 이식세포 혹은 숙주 세포 유래 신경세포가 반대쪽 대뇌 반구의 목표 신경세포와 장거리 신경연결망인 transcallosal projection을 형성하는데 중요한 역할을 함을 보였다(Figure 1).
저산소 허혈 뇌 손상 후에는 신경교질반흔(glial scar)이 형성되는데 뇌 실질에 물리적/분자적 장벽(barrier)으로 작용하여 신경재생을 방해한다. 특히 반응성 별아교세포(reactive astrocytes)는 교질반흔의 중요한 구성요소 중 하나로 신경축삭돌기의 재생과 신경기능 회복을 방해한다[79]. 본 연구에서는 hNSPCs-PGA, PGA 및 vehicle을 각각 저산소 허혈 뇌 손상 부위에 이식하고 8주 후 별아교세포의 표지자인 GFAP 발현을 면역조직화학법으로 분석하였는데, hNSPCs-PGA 이식한 경우 뇌경색증 주위 부분은 GFAP 염색이 크게 증가하였으나 뇌 손상 중앙 부위, 즉 이식으로 새로운 조직이 형성된 부위에는 GFAP가 거의 발현되지 않았다. PGA만 이식한 경우에도 뇌 손상 중앙 부위에서 GFAP 발현이 감소함을 보였고, 반면에 vehicle을 이식한 경우에는 뇌 손상 부위에 GFAP 발현이 크게 증가하였다. 따라서 hNSPCs-PGA 및 PGA를 이식한 경우 뇌 손상 부위에 GFAP-발현 성상 교세포의 침투가 크게 감소하여 신경교질반흔 형성이 감소됨을 보였다. hNSPCs-PGA 이식이 뇌 손상 시 microglia/macrophage의 반응에 미치는 영향을 알아보기 위하여 Iba1, CD68, CD86 및 CD206 항체를 이용하여 면역조직화학적 분석을 시행하였는데, hNSPCs-PGA 및 PGA를 이식한 경우 대조군에 비해 뇌 손상 중앙 부위에서 Iba1, CD68 및 CD86 염색은 감소하였고 CD206 염색은 증가함을 보였다. 따라서 뇌 손상 시 hNSPCs-PGA 및 PGA 이식은 손상 부위에 microglia/macrophage 침투를 감소시키고 세포 분극을 M1 형에서 M2 형으로 변화시켜 신경재생/복구에 도움이 되는 것으로 사료되었다[56,57]. 한편 hNSPCs-PGA 이식 8주 후 뇌 손상 부위에 새롭게 형성된 조직에서 신생혈관생성 여부를 분석하기 위하여 CD31 항체를 이용하여 염색한 결과, PGA 및 vehicle을 이식한 대조군과 비교하여 CD31+ 신생혈관 수가 크게 증가하여 뇌 손상 부위에 hNSPCs-PGA 이식은 인간 NSPC 생착을 위한 구조적 받침대를 제공할 뿐만 아니라 신생혈관생성도 촉진하였다. 저산소 허혈 뇌 손상 부위에 hNSPCs-PGA 이식이 모델동물의 신경학적 기능 및 운동기능에 미치는 영향을 분석하였는데, hNSPCs-PGA 이식군은 이식 5, 7, 9 및 11주 후 신경학적 검사상 vehicle 이식 대조군과 비교하여 유의한 운동-감각기능 호전을 보였고, hNSPCs만 이식한 경우는 이식 5주때에서만 대조군에 비해 기능이 호전되었으며, PGA만 이식한 경우는 대조군과 비교하여 차이가 없었다. Accelerating rotarod 검사에서는 hNSPCs-PGA 이식군은 이식 11주 후 대조군과 비교하여 유의한 운동기능 호전을 보였고, PGA 및 hNSPCs을 이식한 경우는 차이가 없었다. 따라서 저산소 허혈 뇌 손상 부위에 hNSPCs-PGA 이식은 비정상적인 신경기능 장애를 유발함이 없이 운동-감각기능의 호전을 촉진시켰다.
신생아 중증 저산소 허혈 뇌 손상은 광범위한 조직 손상으로 뇌연화증 혹은 대뇌 반구에 큰 뇌경색증 와동을 형성하여 모든 종류의 신경세포들이 손상을 받고, 적절한 신경영양인자, 혈액공급 및 이웃한 신경세포로부터 신경접합신호(synaptic input)들을 받지 못하며, 신경원 및 교세포의 성숙장애와 신경연결망의 변형 등으로 인하여 신경기능 및 행동의 장애를 유발한다[80-82]. 따라서 중증 저산소 허혈 뇌 손상 부위에 신경재생 효과가 우수한 NSPC를 이식하여도 단지 약간의 구조적 및 기능적 호전을 보일 뿐이다. 본 연구에서는 임상적용이 가능한 생분해성 고분자화합물 비계인 PGA와 세포변형을 유발하지 않은 태아 뇌 유래 일차 인간 NSPC 복합체를 뇌 손상 부위에 이식하였는데, 인간 NSPC와 PGA, 이식된 복합체와 숙주 뇌 조직 간 상호작용에 의하여 신경재생 반응이 일어나 이식세포의 생존, 이주 및 분화가 촉진되고, 신경돌기 및 신경축삭돌기의 신전이 촉진되며, 뇌경색증 범위, 신경교질 반흔 형성 및 microglia/macrophage 침투가 감소하고, microglia/macrophage 세포 분극이 변화하고, 신생혈관생성이 촉진되어 신경기능의 호전을 보였다. 이상의 연구 결과로 hNSPCs-PGA 이식은 이식세포에 의한 직접적인 신경세포 재생 및 이식세포와 숙주 신경세포와의 신경접합 형성 등으로 신경복구가 일어나기 보다는 신경보호 효과, 신경가소성 향상, 항염증/면역제어 조절 및 혈관생성촉진 작용 등 복합양식(multimodal)으로 작용하여 뇌 손상에 따른 이차 손상을 막고, 신경돌기 및 신경축삭돌기 신전이 유도되는 것으로 보인다. 천연(natural) 혹은 합성 고분자화합물 비계는 줄기세포의 운송 수단으로 사용될 뿐 아니라 뇌 손상 부위의 간격을 연결하는 안내자 역할을 하고, 뇌 손상 부위의 미세환경을 변화시켜 숙주 뇌 조직에 구조적으로 통합되어 기능적 재생을 유발한다[15,27,76,83-85]. 더욱이 고분자화합물 비계의 세포외기질 성분들은 줄기세포 표면의 수용체에 결합하여 비계와 세포의 부착, 줄기세포의 성장, 이주, 분화 및 생존 등에 중요한 작용을 하고[86-89], 영양분 및 대사산물을 확산시키며, 신경교질 반흔 형성을 방해하고, 신경영양인자 및 혈관생성촉진인자들을 농축시키며, 신경돌기 및 신경축삭돌기의 신전을 촉진시켜 손상된 신경연결망 복구를 유도한다. 또한 최근의 연구에 의하면 electrospinning 방법으로 주사로 주입 가능하고 젤(gel)과 같은 삼차원적인 구조의 nanofibers를 제작하여 인간 NSPC와 함께 허혈성 뇌 손상 부위에 주사할 경우 줄기세포의 ‘stemness’와 생존율을 향상시켜 치료효과를 상승시킴이 보고되었다[77].
따라서 향후 중추신경계 조직과 유사한 기계적 특성과 인간 NSPC 이식에 적합한 리간드(ligand)를 발현하며, 적절한 신경영양인자를 분리시키고 조직재생 능력을 촉진시키며, 염증반응을 효과적으로 조절할 수 있는 우수한 생체재료를 개발하고, 뇌 손상 시 병리적 미세환경을 변화시키는 물질을 발현하는 인간 NSPC와 함께 복합적으로 사용할 경우 보다 효과적으로 저산소 허혈 뇌 손상을 치료할 수 있을 것으로 사료된다.
저산소 허혈 뇌 손상에서 줄기세포 이식 치료효과를 높이고 부작용 발생을 줄이기 위해서는 이식된 기능성 줄기세포의 뇌 손상 부위로의 정확한 이주가 중요한데[22,90], 세포의 유전자 변형 혹은 전 처치 등을 통하여 화학유인물질(chemoattractant)에 대한 수용체를 과발현시켜 세포 이주를 촉진한 경우 이주 효율은 높지 않았고[91-93], 화학유도물질을 목표 부위에 직접 주입한 경우는 화학유도물질 자체가 주입된 부위뿐만 아니라 생체 내 다른 부위에도 존재하는 내인성 물질이어서 정확한 부위로 세포 이주를 유도하기가 쉽지 않았다[94,95]. 그러나 자석을 사용하여 세포를 자성 표적화(magnetic targeting)하는 경우는 위의 문제점을 극복할 수 있는데, 자기장(magnetic field)은 생직교(bioorthogonal; 생화학적 과정에서 다른 생체 분자와는 반응하지 않고, 원하는 분자와만 선택적으로 결합하는 성질)하여 생체 내 고유한 생화학적 반응을 간섭하지 않고 세포 이주를 조절할 수 있기 때문이다[96]. 즉, MNPs로 표시된 세포는 높은 자기장 기울기(gradient) 쪽으로 기계적으로 끌려가게 된다[97-100]. 세포가 조직 내에서 이동하려면 세포 내 MNPs는 세포 주위의 기질 및 마찰력 등의 입체적 장벽(steric barrier)을 극복할 수 있는 충분한 기계적 힘을 발휘할 수 있어야 하는데, 일반적인 페라이트(ferrite; 산화철을 한 성분으로 하는 복합 산화물 및 그 유도체) MNPs는 큰 힘을 발휘하기가 쉽지 않고 특히, 자기장 기울기는 외부 자석으로부터의 거리가 멀수록 감소하기 때문에 신체 깊숙한 곳에 존재하는 세포들은 낮은 자기력을 받게 된다. 따라서 세포의 자성 표적화에 관한 연구는 고형 조직(solid tissue)에서보다는 주로 혈액, 뇌척수액, 안구의 유리 겔(vitreous gel) 및 연골의 활액(synovial fluid)과 같은 액체 조직(liquid tissue) 환경에서 이루어졌고[97-101], 고형 조직에서 세포를 자성 표적화하려면 강한 기계적 힘을 발휘하는 MNPs를 사용하는 것이 중요하다.
본 연구에서는 원격으로 외부 자기장을 이용하여 비침투적 방법으로 고형 조직인 뇌에서 인간 NSPC의 세포 이주를 조절하는 자성 표적화 가능 여부를 실험하였는데, 먼저 충분한 기계적인 힘을 발휘하여 고형 조직 내에서도 세포를 이동시킬 수 있는 15 nm 크기의 ZnMNPs (Zn0.4Fe2.6O4)를 개발하였고, ZnMNPs는 1 Tesla (T) 외부 자기장 하에서 161 emu/g (metal)의 포화 자화(saturation magnetization)에 도달하여 일반적으로 상업용으로 사용하는 페라이트 MNPs (Feridex, TaeJoon Pharm, Seoul, Korea)의 포화 자화보다 약 2.5배 이상 커서 보다 강한 기계적 힘으로 조직 내에서 세포를 끌어당길 수 있음을 보여주었다[101]. 인간 NSPC에 ZnMNPs를 형질 이입(transfection)하기 위하여 ZnMNPs를 poly-L-lysine에 배양 후 줄기세포에 첨가하니 ZnMNPs는 세포 내로 이입(endocytosis)되어 약 90% 이상의 세포들이 ZnMNPs로 표시되었고, 세포 내 엔도솜(endosomes)에 위치함을 보였다. 시험관 내에서 세포의 자성 타겟팅 능력을 평가하기 위하여 ZnMNPs가 형질 이입된 인간 NSPC (ZnMNPs-hNSPCs)와 대조군인 15 nm Fe3O4 MNPs가 형질 이입된 인간 NSPC (MNPs-hNSPCs), Feridex가 형질 이입된 인간 NSPC (Feridex-hNSPCs) 및 MNP로 표시되지 않은 hNSPCs를 실제 뇌 조직과 유사한 밀도를 가지는 agarose gel로 채워진 capillary tube의 한 쪽 끝에 놓고, 반대편 끝에는 1 T 네오디뮴(neodymium, NdFeB) 자석을 배치한 후 시간에 따른 세포의 이동 거리와 속도를 측정하였는데, ZnMNPs-hNSPCs가 가장 빠른 속도로 멀리 움직였고, MNPshNSPCs와 Feridex-hNSPCs는 천천히 짧은 거리를 이동하였으며, hNSPCs는 거의 움직이지 않았다. 따라서 인간 NSPC의 자성 표적화 능력을 높이고 뇌 속에서 세포 이주를 조절하기 위해서는 높은 자기모멘트(magnetic moment)를 가지는 ZnMNPs를 사용함이 필수적이었다.
실제 쥐의 뇌 속에서 ZnMNPs-hNSPCs의 자성 표적화 정도를 시험하기 위하여 ZnMNPs-hNSPCs를 직접 측 뇌실(lateral ventricle) 내로 이식하고 5일 후 이식세포의 이주 경로를 조사한 결과, 이식세포는 측 뇌실 및 뇌실 하 영역(subventricular zone)에서 rostral migratory stream (RMS)을 따라 쥐의 후구(olfactory bulb)로 이동하였고, 또한 뇌량을 따라 대뇌 백질의 바깥섬유막으로 이주함이 관찰되었다. 일반적으로 뇌실 하 영역에 존재하는 내인성 NSPC는 뇌의 시공간 신호(spatiotemporal signal)에 반응하여 RMS, 후구, 뇌량 및 뇌 백질 부위로 이주하는데[102], 상기 실험 결과로 ZnMNPs-hNSPCs를 뇌 이식할 경우 생체 내 내인성 NSPC와 동일한 본태성 세포 이주 능력을 보유하고 있음을 나타냈다. 반면에 ZnMNPs-hNSPCs를 측 뇌실에 이식하고 쥐의 머리 부위에 1 T 네오디뮴 외부 자석을 부착하여 자기장을 걸어준 경우, 이식세포는 측 뇌실 부위에서 외부 자석이 위치한 대뇌피질 및 피질 하 영역(subcortex)으로 이주함을 보였다. 대조군 MNPs-hNSPCs를 측 뇌실 부위에 이식하고 외부 자석을 부착한 경우, 이식세포는 주로 이식 부위에 존재하고 일부 세포는 대뇌피질 하 영역의 하부에 도달함을 보였다. 따라서 쥐의 뇌 속에서 이식된 ZnMNPs-hNSPCs는 사용자가 원하는 방향으로 세포 이주를 유도하여 자성 표적화가 가능함을 보였다[101]. 또한 ZnMNPs-hNSPCs의 자성 표적화 능력을 정상 뇌 조직 환경뿐만 아니라 중대뇌동맥 폐색(middle cerebral artery occlusion) 유도 뇌졸중 모델에서도 적용하여 보았는데, 뇌졸중 시 혈관-뇌 장벽의 일시적 붕괴로 인하여 투과성이 증가하므로[103] 오른쪽 중대뇌동맥 폐쇄하여 뇌졸중을 유도하고 1 T 네오디뮴 외부 자석을 쥐의 머리에 부착하였다. 24시간 후 ZnMNPs-hNSPCs을 오른쪽 내경동맥(internal carotid artery)에 주입하고 주입 전, 주입 1일 및 3주 후 뇌 MRI 검사와 면역조직화학법검사를 실시한 결과, 이식세포는 혈관으로부터 뇌의 복측으로 들어가서 외부 자석 부착 방향으로 이동하여 뇌 손상 중심 부위, 뇌경색증 주위 대뇌피질, 선조체 및 대뇌 백질의 바깥섬유막 부위로 이동함을 보였다. 반면, 대조군에서는 외부 자석을 부착하지 않고 같은 수의 ZnMNPs-hNSPCs을 내경동맥에 주입하였는데, 상기와 같은 세포 이주를 관찰할 수 없었다. 또한 BLI 기법으로 생체 내 살아있는 이식세포의 생착률을 비교한 결과 실험군은 대조군에 비해 약 60배 뇌 손상 부위에 생착률이 증가하여 혈관을 통하여 비침습적 방법으로 세포를 이식하여도 직접 뇌 손상 부위로 이식한 경우와 유사한 세포 생착률을 보였다.
ZnMNPs-hNSPCs은 세포 이주 및 이동이 촉진되었을 뿐만 아니라 세포분화 조건 하에서 신경세포로의 분화도 촉진되었는데, 시험관 내에서 대조군 MNPs-hNSPCs 및 hNSPCs과 비교하여 신경세포의 표지자인 TUJ1+ 및 MAP2+ 세포의 비율은 크게 증가하였고, Nestin+ 및 GFAP+ 세포의 비율은 유의하게 감소하였으며, 신경세포로의 분화를 유발하는 전사인자인 NEUROG2 의 발현도 크게 증가함을 보였다. ZnMNPs-hNSPCs를 정상 뇌뿐만 아니라 뇌졸중 뇌에 이식한 경우도 상기 대조군에 비해 TUJ1 발현이 약 4배 정도 증가하였고, NGF 및 NTF3 발현도 대조군과 비교하여 약 2.5배 및 2.2배 증가함을 보였다. 따라서 ZnMNPs가 형질 이입된 인간 NSPC는 신경세포로의 분화가 촉진되고 신경영양인자의 발현도 크게 증가하였다. 세포분화 기전을 연구하기 위하여 ZnMNPs-hNSPCs에 Wnt 신호전달경로 차단제인 Dikkopf1 (DKK1)을 처리하니 신경세포로의 분화와 신경영양인자 발현이 유의하게 감소하였다. Western blot 및 quantitative polymerase chain reaction 검사 결과 ZnMNPs-hNSPCs와 DKK1-treated ZnMNPs-hNSPCs은 Wnt 신호전달경로의 upstream인 zinc finger 전사인자인 ZIC1 및 ZIC2는 증가되어 있었고, Wnt 신호전달 리간드인 WNT1 및 WNT3a 도 증가함을 보였다. 그러나 ZnMNPs-hNSPCs은 Wnt 신호전달경로의 downstream인 β-catenin 및 axis inhibition protein 2 (AXIN2 ) 발현도 증가하였지만, DKK1-treated ZnMNPs-hNSPCs은 상기 분자 발현이 증가하지 않았다. 따라서 ZnMNPs는 zinc 매개 Wnt 신호전달경로를 활성화시켜 인간 NSPC의 신경세포로의 분화와 신경영양인자 분비를 촉진시킴을 확인하였다.
뇌졸중 모델에서 ZnMNPs-hNSPCs를 내경동맥을 통하여 주입하고 외부 자석으로 자성 표적화하였을 때 신경기능에 미치는 영향을 분석하기 위하여 세포 주입 3주 후 asymmetric cylinder 검사를 실시하였는데, vehicle 및 hNSPCs를 주입한 대조군과 비교하여 앞발의 운동기능이 유의하게 회복되었고, 면역조직화학검사상 이식된 ZnMNPs-hNSPCs는 신경세포로 분화하고 뇌 손상 주위 숙주 뇌세포와 신경접합을 형성하여 숙주 뇌 조직에 통합됨을 확인하였다[101].
이상의 연구 결과로 강력한 자기 모멘트를 가지는 ZnMNPs는 중추신경계와 같은 고형 조직 환경에서 비침습적으로 세포를 자성 타겟팅하여 원하는 방향으로 이주를 가능케 하였으며, 인간 NSPC에 쉽게 형질 이입되어 신경세포로의 분화와 신경영양인자의 분비를 촉진시켜 뇌 손상 부위에 비침습적 방법으로 주입되었을 때 효과적으로 신경기능 개선을 유도하였고, 뇌 MRI 검사 등으로 생체 내 이식세포의 이주, 생착 등의 행동을 영상화하여 추적 가능케 한다. 따라서 나노공학적 융합연구를 통하여 ZnMNPs가 형질 이입된 인간 NSPC 이식은 향후 다양한 퇴행성 신경계질환에 대한 세포치료 시치료적 유효성을 획기적으로 개선시키는 기반 연구가 될 것이다.
신경발달 관련 전사인자인 NEUROG2-hNSPC를 저산소 허혈 뇌 손상 부위에 이식한 경우, 이식세포는 주로 신경세포로 분화하고 다양한 신경영양인자들을 활발히 분비하여 뇌 손상 부위의 병리적 미세환경을 변화시켜, 신경세포의 생존 및 신경가소성을 현저히 증가시키고 신경기능 회복이 크게 촉진되었으며, 대표적인 항염증 싸이토카인인 IL-10 발현 인간 NSPC를 뇌 손상 부위에 이식한 경우, 항염증/면역조절, 신경보호 및 혈관생성 작용 등 다양한 기전으로 신경 재생 및 복구를 유발하여 향후 신생아 저산소 허혈 뇌 손상 환아에서 인간 NSPC 기반 세포-유전자 치료 적용 시 치료적 유효성을 크게 향상시킬 것으로 기대된다. 또한 인간 NSPC에 TNF-α를 전 처치할 경우 NF-κB 신호전달을 통하여 cIAP2 유전자 발현이 증가하여 뇌 손상 부위에 이식된 세포 생존율이 증가하고, 역시 NF-κB 신호전달을 통하여 CXCL1 유전자 발현이 증가하여 항염증 작용에 의한 신경보호 효과를 보이며, TNF-α 전 처치 인간 NSPC를 뇌 손상 부위에 이식한 경우 뇌경색증 범위의 감소와 신경기능 개선을 보였다. 중증 저산소 허혈 뇌 손상은 광범위한 조직 손상으로 모든 종류의 신경세포들이 손상을 받고, 적절한 신경영양인자, 혈액공급 및 이웃한 신경세포로부터 신경접합신호를 받지 못하기 때문에 신경재생 효과가 우수한 신경줄기세포를 이식하여도 단지 약간의 구조적 및 기능적 호전을 보이는 한계가 있다. 따라서, 임상적용이 가능한 생분해성 고분자화합물 비계인 PGA와 인간 NSPC 복합체를 뇌 손상 부위에 이식한 경우, 줄기세포와 PGA 및 이식된 복합체와 숙주 뇌 조직 간에 상호작용에 의하여 신경재생 반응이 일어나 이식세포의 생존, 이주 및 분화가 촉진되고, 신경돌기 및 축삭돌기의 신전이 촉진되며, 뇌경색증 범위, 신경교질 반흔 형성 및 microglia/macrophage 침투가 감소하고, microglia/macrophage 세포 분극이 변화하고, 신생혈관생성이 촉진되어 신경기능의 호전을 보였다. 한편 강력한 자기모멘트를 가지는 ZnMNPs를 형질 이입한 인간 NSPC는 뇌와 같은 고형 조직 환경에서 비침습적으로 세포를 자성 표적화하여 원하는 방향으로 이주를 가능케 하였으며, 신경세포로의 분화와 신경영양인자의 분비를 촉진시켜 뇌 손상 부위에 비침습적 방법으로 주입되었을 때 효과적으로 신경기능 개선을 유도하였고, 뇌 MRI 검사 등으로 생체 내 이식세포의 이주, 생착 등의 행동을 영상화하여 추적 가능케하였다. 따라서 나노공학적 융합연구를 통하여 ZnMNPs가 형질 이입된 인간 NSPC 이식은 향후 다양한 퇴행성 신경계질환에 대한 세포치료 시 치료적 유효성을 획기적으로 개선시키는 기반 연구가 될 것이다.
신생아 저산소 허혈 뇌 손상은 높은 사망률과 신경발달학적 후유증을 남기지만 현재까지 많은 연구가 진행되어 왔음에도 불구하고 저체온증 유도 시 제한적 신경보호 효과 외에는 특별한 치료법이 없다. NSPC는 미분화 상태로 계속 증식하는 자가갱신을 보이고, 한 개의 줄기세포로부터 다양한 신경원 및 교세포로 분화하는 분화의 다능성을 보이는 세포로 정의한다. 신생아 저산소성 허혈성 뇌 손상동물 모델에 인간 NSPC를 이식한 경우 이식세포는 뇌 손상 부위에 생착하고 광범위하게 이주하여 신경원 및 교세포로 분화하고, 다양한 신경전달물질을 발현하며, 숙주 신경세포와 신경접합을 형성하여 신경기능의 호전을 보였고, 비정상적인 신경행동 소견이나 뇌종양 형성 등은 보이지 않았다. 그러나 NSPC를 뇌 손상 부위에 이식한 경우 이식세포의 생착, 생존 및 통합률이 불량하고, 특이 신경세포로의 분화가 잘 안 되거나 주로 신경교세포로 분화하며, 해부학적/기능적으로 정밀한 신경연결망 형성도 이루어지지 않아 현재로는 NSPC 이식 치료효과가 제한적이다. 따라서 치료적 유효성이 획기적으로 개선된 기능성 NSPC를 개발하기 위하여 줄기세포 치료 기전 연구와 유전공학, 조직공학 및 나노공학적 융복합 연구를 통한 차세대 세포치료법 개발이 필요한데, 본 종설에서는 그동안 융복합 연구를 통해 치료적 유효성이 향상된 인간 NSPC 기반 차세대 세포치료법 개발 결과를 약술하였다.
Acknowledgments
이 논문에 서술된 연구들은 한국연구재단과 보건의료기술 연구 개발사업 지원을 받아 수행되었습니다(NRF; 2013M3A9B4076545, HI14C1564, HI16C1089). 기술적 도움을 주신 연세-칼자이스 이미징 센터에 감사 드립니다.
Figure 1.
Transplantation (Tx) of human nucleus pulposus cell (hNPC)-poly (glycolic acid) (PGA) complexes into the infarction cavity of a hypoxic-ischemic (HI) brain injury. (A) Two weeks following Tx of the hNPC-PGA complexes (an arrow) into the infarction cavity indicated by an asterisk; human nuclei (hNuc)+donor-derived cells (green) show robust engraftment within the injured area. The PGA fibers begin to biodegrade (green strands, arrowheads). (B-G) Some human neural stem/progenitor cell (hNSPC)+donor-derived cells (red in B, D, E, G) presented co-localization with neurofilament (NF) (blue arrows in B, C, D) with either extended neuronal processes (white arrows in C, D; n=15) or glial fibrillary acidic protein (GFAP) (white arrows in E, F, G; n=15). The PGA fibers are indicated by arrowheads in B-G. The tract tracer biotinylated dextran amine conjugated with fluorescein (BDA-FITC) was injected into the contralateral intact cortex at 10 weeks following implantation of the hNPC-PGA complex into the infarction cavity. Axonal projections were labeled in green with fluorescein and can be visualized from the cerebral cortex as well as the external capsule (EC) of the intact hemisphere, projecting through the corpus callosum (CC) and toward the implantation site of the hNPC-PGA complex within the injured ipsilateral cortex and penumbra (H). Some BDA-FITC+anterograde-labeled processes from the neurons in the contralateral intact hemisphere were observed to sprout their axons toward the implantation site of the hNPC-PGA complex (H). The BDA-FITC+retrograde-labeled cell body and cellular processes of a neuron-like cell in the ipsilateral cortical penumbra indicated by an asterisk (in H) are well visualized at a higher magnification (I). The data shown are representative images. Scale bars (A, H: 500 μm; B, E: 20 μm).
REFERENCES
2. Volpe JJ. Neonatal encephalopathy: an inadequate term for hypoxic- ischemic encephalopathy. Ann Neurol 2012;72:156–66.


3. Douglas-Escobar M, Weiss MD. Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr 2015;169:397–403.


4. Conti L, Cattaneo E. Neural stem cell systems: physiological players or in vitro entities? Nat Rev Neurosci 2010;11:176–87.



7. Ourednik V, Ourednik J, Flax JD, Zawada WM, Hutt C, Yang C, et al. Segregation of human neural stem cells in the developing primate forebrain. Science 2001;293:1820–4.


8. Svendsen CN, ter Borg MG, Armstrong RJ, Rosser AE, Chandran S, Ostenfeld T, et al. A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Methods 1998;85:141–52.


9. Vescovi AL, Parati EA, Gritti A, Poulin P, Ferrario M, Wanke E, et al. Isolation and cloning of multipotential stem cells from the embryonic human CNS and establishment of transplantable human neural stem cell lines by epigenetic stimulation. Exp Neurol 1999;156:71–83.


10. Palmer TD, Ray J, Gage FH. FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci 1995;6:474–86.


11. Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 1992;255:1707–10.


12. Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci 2006;7:395–406.



13. Abematsu M, Tsujimura K, Yamano M, Saito M, Kohno K, Kohyama J, et al. Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J Clin Invest 2010;120:3255–66.



14. Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci U S A 2004;101:11839–44.



15. Park KI, Teng YD, Snyder EY. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat Biotechnol 2002;20:1111–7.



16. Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Muller FJ, Loring JF, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A 2009;106:13594–9.



17. Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature 2005;436:266–71.



18. Tamaki SJ, Jacobs Y, Dohse M, Capela A, Cooper JD, Reitsma M, et al. Neuroprotection of host cells by human central nervous system stem cells in a mouse model of infantile neuronal ceroid lipofuscinosis. Cell Stem Cell 2009;5:310–9.


19. Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, et al. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain 2011;134:1777–89.




20. Gage FH, Temple S. Neural stem cells: generating and regenerating the brain. Neuron 2013;80:588–601.


21. Daadi MM, Davis AS, Arac A, Li Z, Maag AL, Bhatnagar R, et al. Human neural stem cell grafts modify microglial response and enhance axonal sprouting in neonatal hypoxic-ischemic brain injury. Stroke 2010;41:516–23.



22. Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A 2004;101:18117–22.



23. Park KI, Hack MA, Ourednik J, Yandava B, Flax JD, Stieg PE, et al. Acute injury directs the migration, proliferation, and differentiation of solid organ stem cells: evidence from the effect of hypoxia-ischemia in the CNS on clonal "reporter" neural stem cells. Exp Neurol 2006;199:156–78.


24. Park KI, Himes BT, Stieg PE, Tessler A, Fischer I, Snyder EY. Neural stem cells may be uniquely suited for combined gene therapy and cell replacement: evidence from engraftment of Neurotrophin-3-expressing stem cells in hypoxic-ischemic brain injury. Exp Neurol 2006;199:179–90.


25. Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders: time for clinical translation? J Clin Invest 2010;120:29–40.



27. Orive G, Anitua E, Pedraz JL, Emerich DF. Biomaterials for promoting brain protection, repair and regeneration. Nat Rev Neurosci 2009;10:682–92.



28. Hofstetter CP, Holmstrom NA, Lilja JA, Schweinhardt P, Hao J, Spenger C, et al. Allodynia limits the usefulness of intraspinal neural stem cell grafts: directed differentiation improves outcome. Nat Neurosci 2005;8:346–53.



29. Hicks AU, Lappalainen RS, Narkilahti S, Suuronen R, Corbett D, Sivenius J, et al. Transplantation of human embryonic stem cellderived neural precursor cells and enriched environment after cortical stroke in rats: cell survival and functional recovery. Eur J Neurosci 2009;29:562–74.


30. Nakagomi N, Nakagomi T, Kubo S, Nakano-Doi A, Saino O, Takata M, et al. Endothelial cells support survival, proliferation, and neuronal differentiation of transplanted adult ischemiainduced neural stem/progenitor cells after cerebral infarction. Stem Cells 2009;27:2185–95.


31. Park KI, Goo K, Jung K, Kim M, Kim IS, Yun S, et al. Therapeutic application of neural stem cells for neonatal hypoxic-ischemic brain injury. Neonatal Med 2013;20:343–53.

32. Wilkinson G, Dennis D, Schuurmans C. Proneural genes in neocortical development. Neuroscience 2013;253:256–73.


33. Serre A, Snyder EY, Mallet J, Buchet D. Overexpression of basic helix-loop-helix transcription factors enhances neuronal differentiation of fetal human neural progenitor cells in various ways. Stem Cells Dev 2012;21:539–53.


34. Yi SH, Jo AY, Park CH, Koh HC, Park RH, Suh-Kim H, et al. Mash1 and neurogenin 2 enhance survival and differentiation of neural precursor cells after transplantation to rat brains via distinct modes of action. Mol Ther 2008;16:1873–82.


35. Lee IS, Koo KY, Jung K, Kim M, Kim IS, Hwang K, et al. Neurogenin-2-transduced human neural progenitor cells attenuate neonatal hypoxic-ischemic brain injury. Transl Res 2017;183:121–36.


36. Hagberg H, Mallard C, Ferriero DM, Vannucci SJ, Levison SW, Vexler ZS, et al. The role of inflammation in perinatal brain injury. Nat Rev Neurol 2015;11:192–208.



37. Banjara M, Ghosh C. Sterile neuro inflammation and strategies for therapeutic intervention. Int J Inflam 2017;2017:8385961.




38. Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature 2003;422:688–94.



39. Einstein O, Fainstein N, Vaknin I, Mizrachi-Kol R, Reihartz E, Grigoriadis N, et al. Neural precursors attenuate autoimmune encephalomyelitis by peripheral immunosuppression. Ann Neurol 2007;61:209–18.


40. Lee IS, Jung K, Kim IS, Lee H, Kim M, Yun S, et al. Human neural stem cells alleviate Alzheimer-like pathology in a mouse model. Mol Neurodegener 2015;10:38.



41. Kwilasz AJ, Grace PM, Serbedzija P, Maier SF, Watkins LR. The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology 2015;96:55–69.


42. de Bilbao F, Arsenijevic D, Moll T, Garcia-Gabay I, Vallet P, Langhans W, et al. In vivo over-expression of interleukin-10 increases resistance to focal brain ischemia in mice. J Neurochem 2009;110:12–22.


43. Ooboshi H, Ibayashi S, Shichita T, Kumai Y, Takada J, Ago T, et al. Postischemic gene transfer of interleukin-10 protects against both focal and global brain ischemia. Circulation 2005;111:913–9.


44. Grilli M, Barbieri I, Basudev H, Brusa R, Casati C, Lozza G, et al. Interleukin-10 modulates neuronal threshold of vulnerability to ischaemic damage. Eur J Neurosci 2000;12:2265–72.


45. Vila N, Castillo J, Davalos A, Esteve A, Planas AM, Chamorro A. Levels of anti-inflammatory cytokines and neurological worsening in acute ischemic stroke. Stroke 2003;34:671–5.


46. Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev 2011;91:461–553.


47. Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 2007;8:57–69.



48. Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr Med Chem 2007;14:1189–97.


49. Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med 2011;17:796–808.




50. Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 2007;10:1387–94.



51. Kwon MJ, Kim J, Shin H, Jeong SR, Kang YM, Choi JY, et al. Contribution of macrophages to enhanced regenerative capacity of dorsal root ganglia sensory neurons by conditioning injury. J Neurosci 2013;33:15095–108.



52. Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci 2013;16:1211–8.




53. Thored P, Heldmann U, Gomes-Leal W, Gisler R, Darsalia V, Taneera J, et al. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia 2009;57:835–49.


54. Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A 2003;100:13632–7.



55. Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, et al. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke 2007;38:146–52.


56. David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 2011;12:388–99.


57. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008;8:958–69.




58. Rosenblum S, Smith TN, Wang N, Chua JY, Westbroek E, Wang K, et al. BDNF pretreatment of human embryonic-derived neural stem cells improves cell survival and functional recovery after transplantation in hypoxic-ischemic stroke. Cell Transplant 2015;24:2449–61.


60. Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H, et al. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain 2006;129:2734–45.




61. Lee HJ, Kim MK, Kim HJ, Kim SU. Human neural stem cells genetically modified to overexpress Akt1 provide neuroprotection and functional improvement in mouse stroke model. PLoS One 2009;4:e5586.



62. Sakata H, Niizuma K, Wakai T, Narasimhan P, Maier CM, Chan PH. Neural stem cells genetically modified to overexpress cu/zn-superoxide dismutase enhance amelioration of ischemic stroke in mice. Stroke 2012;43:2423–9.



63. Wang F, Kameda M, Yasuhara T, Tajiri N, Kikuchi Y, Liang HB, et al. GDNF-pretreatment enhances the survival of neural stem cells following transplantation in a rat model of Parkinson's disease. Neurosci Res 2011;71:92–8.


64. Sakata H, Narasimhan P, Niizuma K, Maier CM, Wakai T, Chan PH. Interleukin 6-preconditioned neural stem cells reduce ischaemic injury in stroke mice. Brain 2012;135:3298–310.




65. Schwabe RF, Brenner DA. Mechanisms of liver injury. I. TNFalpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol 2006;290:G583–9.

66. Turrin NP, Rivest S. Tumor necrosis factor alpha but not interleukin 1 beta mediates neuroprotection in response to acute nitric oxide excitotoxicity. J Neurosci 2006;26:143–51.



67. Bernardino L, Agasse F, Silva B, Ferreira R, Grade S, Malva JO. Tumor necrosis factor-alpha modulates survival, proliferation, and neuronal differentiation in neonatal subventricular zone cell cultures. Stem Cells 2008;26:2361–71.


68. Peng H, Whitney N, Wu Y, Tian C, Dou H, Zhou Y, et al. HIV-1-infected and/or immune-activated macrophage-secreted TNFalpha affects human fetal cortical neural progenitor cell proliferation and differentiation. Glia 2008;56:903–16.



69. Lan X, Chen Q, Wang Y, Jia B, Sun L, Zheng J, et al. TNF-α affects human cortical neural progenitor cell differentiation through the autocrine secretion of leukemia inhibitory factor. PLoS One 2012;7:e50783.



71. Kim M, Jung K, Kim IS, Lee IS, Ko Y, Shin JE, et al. TNF-α induces human neural progenitor cell survival after oxygen-glucose deprivation by activating the NF-κB pathway. Exp Mol Med 2018;50:14.




72. Garcia GE, Xia Y, Chen S, Wang Y, Ye RD, Harrison JK, et al. NFkappaB-dependent fractalkine induction in rat aortic endothelial cells stimulated by IL-1beta, TNF-alpha, and LPS. J Leukoc Biol 2000;67:577–84.


73. Fraticelli P, Sironi M, Bianchi G, D'Ambrosio D, Albanesi C, Stoppacciaro A, et al. Fractalkine (CX3CL1) as an amplification circuit of polarized Th1 responses. J Clin Invest 2001;107:1173–81.



74. Zujovic V, Benavides J, Vige X, Carter C, Taupin V. Fractalkine modulates TNF-alpha secretion and neurotoxicity induced by microglial activation. Glia 2000;29:305–15.


75. Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 2006;9:917–24.



76. Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A 2002;99:3024–9.



77. Lee S, Yun S, Park KI, Jang JH. Sliding fibers: slidable, injectable, and gel-like electrospun nanofibers as versatile cell carriers. ACS Nano 2016;10:3282–94.


78. Skop NB, Calderon F, Cho CH, Gandhi CD, Levison SW. Improvements in biomaterial matrices for neural precursor cell transplantation. Mol Cell Ther 2014;2:19.



80. Fitch MT, Doller C, Combs CK, Landreth GE, Silver J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J Neurosci 1999;19:8182–98.



81. Stone BS, Zhang J, Mack DW, Mori S, Martin LJ, Northington FJ. Delayed neural network degeneration after neonatal hypoxiaischemia. Ann Neurol 2008;64:535–46.



82. Barrett RD, Bennet L, Davidson J, Dean JM, George S, Emerald BS, et al. Destruction and reconstruction: hypoxia and the developing brain. Birth Defects Res C Embryo Today 2007;81:163–76.


83. Huang YC, Huang YY. Biomaterials and strategies for nerve regeneration. Artif Organs 2006;30:514–22.


84. Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, et al. Longdistance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 2012;150:1264–73.



85. Zeng X, Zeng YS, Ma YH, Lu LY, Du BL, Zhang W, et al. Bone marrow mesenchymal stem cells in a three-dimensional gelatin sponge scaffold attenuate inflammation, promote angiogenesis, and reduce cavity formation in experimental spinal cord injury. Cell Transplant 2011;20:1881–99.


86. Olson HE, Rooney GE, Gross L, Nesbitt JJ, Galvin KE, Knight A, et al. Neural stem cell- and Schwann cell-loaded biodegradable polymer scaffolds support axonal regeneration in the transected spinal cord. Tissue Eng Part A 2009;15:1797–805.



87. Cholas RH, Hsu HP, Spector M. The reparative response to crosslinked collagen-based scaffolds in a rat spinal cord gap model. Biomaterials 2012;33:2050–9.


88. Hejcl A, Sedy J, Kapcalova M, Toro DA, Amemori T, Lesy P, et al. HPMA-RGD hydrogels seeded with mesenchymal stem cells improve functional outcome in chronic spinal cord injury. Stem Cells Dev 2010;19:1535–46.


89. Lee KY, Kong HJ, Mooney DJ. Quantifying interactions between cell receptors and adhesion ligand-modified polymers in solution. Macromol Biosci 2008;8:140–5.


90. Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 2009;4:206–16.


91. Cheng Z, Ou L, Zhou X, Li F, Jia X, Zhang Y, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther 2008;16:571–9.


92. Rosova I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 2008;26:2173–82.



93. Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation 2003;108:863–8.


94. Purcell BP, Elser JA, Mu A, Margulies KB, Burdick JA. Synergistic effects of SDF-1α chemokine and hyaluronic acid release from degradable hydrogels on directing bone marrow derived cell homing to the myocardium. Biomaterials 2012;33:7849–57.



95. Park JS, Rhau B, Hermann A, McNally KA, Zhou C, Gong D, et al. Synthetic control of mammalian-cell motility by engineering chemotaxis to an orthogonal bioinert chemical signal. Proc Natl Acad Sci U S A 2014;111:5896–901.



96. Shin TH, Cheon J. Synergism of nanomaterials with physical stimuli for biology and medicine. Acc Chem Res 2017;50:567–72.


97. Riegler J, Liew A, Hynes SO, Ortega D, O'Brien T, Day RM, et al. Superparamagnetic iron oxide nanoparticle targeting of MSCs in vascular injury. Biomaterials 2013;34:1987–94.


98. Landazuri N, Tong S, Suo J, Joseph G, Weiss D, Sutcliffe DJ, et al. Magnetic targeting of human mesenchymal stem cells with internalized superparamagnetic iron oxide nanoparticles. Small 2013;9:4017–26.


99. Vandergriff AC, Hensley TM, Henry ET, Shen D, Anthony S, Zhang J, et al. Magnetic targeting of cardiosphere-derived stem cells with ferumoxytol nanoparticles for treating rats with myocardial infarction. Biomaterials 2014;35:8528–39.


100. Tukmachev D, Lunov O, Zablotskii V, Dejneka A, Babic M, Sykova E, et al. An effective strategy of magnetic stem cell delivery for spinal cord injury therapy. Nanoscale 2015;7:3954–8.


101. Yun S, Shin TH, Lee JH, Cho MH, Kim IS, Kim JW, et al. Design of magnetically labeled cells (mag-cells) for in vivo control of stem cell migration and differentiation. Nano Lett 2018;18:838–45.









