 |
 |
- Search
| Neonatal Med > Volume 30(2); 2023 > Article |
|
Abstract
Purpose
In most neonatal intensive care units, echocardiography is performed at the bedside. The lack of closure of interatrial communications (IACs) in the oval fossa can affect mortality and morbidity. This study aimed to establish the rate of spontaneous closure of IACs diagnosed in the neonatal period and predict the need for subsequent evaluation according to various factors, including defect size and shape.
Methods
The study group comprised 1,242 newborns admitted between March 2017 and December 2020. We judged the closure of the IACs and evaluated the risk factors of each. Patients were classified into the absence or presence of IACs. The morphology of the IACs was classified into atrial septal defects, patent foramen ovale, and multiple holes.
Results
A total of 317 patients (131 female, 186 male) were enrolled in the study, and the average defect size was 3.0┬▒1.0 mm. An overall spontaneous closure rate of 89.9% was observed during an average follow-up interval of 5.1┬▒1.1 months. When multiple Cox regression analyses were performed to analyze the risk factors for spontaneous closure, initial defect size was a significant factor. The optimal cut-off value for predicting spontaneous closure of the IAC was 3.05 mm, and the area under the receiver operating characteristic curve was 0.625 (95% confidence interval, 0.520 to 0.729). Preterm infants had a larger defect size corrected for birth weight and a higher closure rate than term infants.
In most neonatal intensive care units (NICUs), echocardiography is performed at bedside to assess hemodynamic status. Even if interatrial shunts in newborns are frequently encountered, due to limited knowledge of their natural course, inappropriately numerous examinations are being performed cost-effectively.
Interatrial communications (IACs) in the oval fossa is responsible for communication between the systemic and pulmonary circulations. Interatrial shunt extent and direction are related to defect size and relative ventricular compliance, which, if not closed, can affect mortality and morbidity. Campbell reported a low mortality rate in the first two decades of life (0.6% and 0.7% per year, respectively), increasing to 7.5% per year in the sixth decade related to pulmonary hypertension and congestive heart failure [1].
However, even if defect size is small, it can cause several morbidities such as stroke and pulmonary embolism in adulthood. Several studies have shown that patent foramen ovale (PFO) is highly prevalent in cases of cryptogenic young adult stroke [2-5]. The natural history of IACs varies according to the anatomic form, but some studies suggest that the initial diameter is the main predictor of spontaneous closure [6].
This study aimed to establish the rate of spontaneous closure of IACs in the oval fossa diagnosed in the neonatal period and predict the need for subsequent evaluation according to various factors, including defect size and shape.
The study group comprised 1,242 newborns admitted to the NICU of Soonchunhyang University Bucheon Hospital between March 2017 and December 2020. This study was approved by the Institutional Review Board of Soonchunhyang University Bucheon Hospital, which waived the requirement for informed consent (2022-08-022). In this retrospective study, we included patients diagnosed with IACs in the oval fossa during the 4th week of life that were subsequently observed until they spontaneously closed. The medical records and two-dimensional echocardiographic data of all patients were reviewed. The demographic and clinical variables recorded included gestational age, birth weight, age at diagnosis, sex, diagnosis during hospitalization, and duration of hospitalization. Infants with concomitant heart lesions were also excluded. Infants who died during hospitalization, were transferred to another hospital, whose data were incomplete, did not attend follow-up, or underwent echocardiography after 7 months of age were excluded.
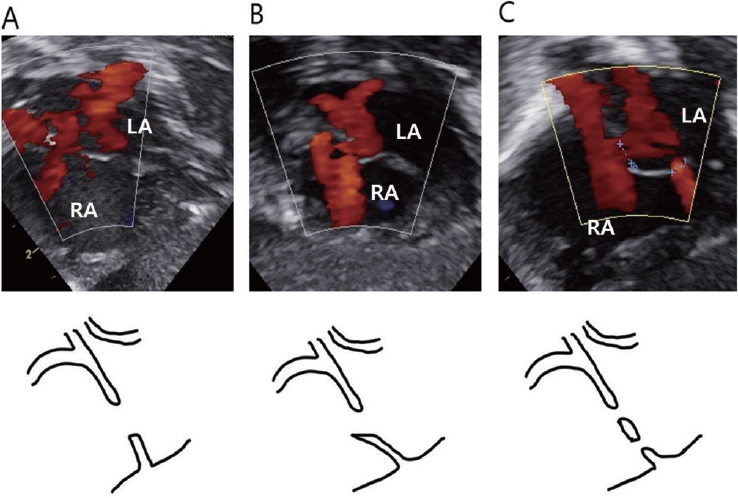
Echocardiography was performed in cases of premature birth, respiratory diseases, cyanosis, pathologic cardiac murmur, and other congenital anomalies. Serial echocardiography was performed according to each patientŌĆÖs clinical course. In most cases, the first examination was performed within 1 week after birth, and the timing of subsequent examinations was determined according to the results. Patients with only IAC without other lesions were followed up at 6 months or at 6 months of corrected age in term infants and preterm infants, respectively. Echocardiographic evaluations were performed by a pediatric cardiologist (S.H.L.). The defect diameter on two-dimensional echocardiography and width of the color flow jet were measured in the subcostal view. The morphology of the IACs was also studied and classified into atrial septal defects (ASDs), PFO, and multiple holes. The secundum ASD (also termed oval fossa defect) is located within the true septum. The defect margin was clear cut, and the shunt flow and interatrial septum (IAS) were almost vertical. PFO is a tunnel-like passageway between the free edge of the overlapping ovale fossa valve and its muscular rim. This type includes cases in which adhesion does not occur or a shunt occurs due to a stretched secondary septum. The shunt flow and IAS angle were <90┬░. If there were more than two holes, the patient was classified as having multiple holes (Figure 1). In the multiplehole group, overall size was determined by combining these sizes. The presence of other shunt lesions and accompanying diseases on echocardiography were also investigated.
The outcome categories were classified as the absence or presence of IACs. We judged IAC closure at 5 to 7 months of age in term infants and corrected age in preterm infants, then evaluated risk factors including gestational age, birth weight, age at diagnosis, sex, diagnosis of hospitalization, duration of hospitalization, defect size, morphological subtypes, and concomitant heart disease.
The statistical analyses were performed using the Rex Excelbased statistical analysis software version 3.6.0 (RexSoft; http://rexsoft.org/) based on R version 4.0.0 (R Foundation for Statistical Computing). A receiver operating characteristic (ROC) curve analysis was performed using SPSS software version 12.0 (SPSS). Continuous variables are summarized as mean┬▒standard deviation or median (range), while categorical data are shown as number (percentage). The Mann-Whitney U-test was used to compare the continuous demographic data. The chi-squared test and FisherŌĆÖs exact test were used to compare proportions between groups. Cox regression analysis was used to identify predictors of spontaneous IAC closure. The independent variables entered into the regression models were gestational age, birth weight, age at diagnosis, sex, diagnosis of hospitalization, duration of hospitalization, defect size, defect size corrected for birth weight, morphological subtypes, and concomitant heart disease. The effects are reported as hazard ratios and 95% confidence intervals (CI), and values of P<0.05 were considered significant.
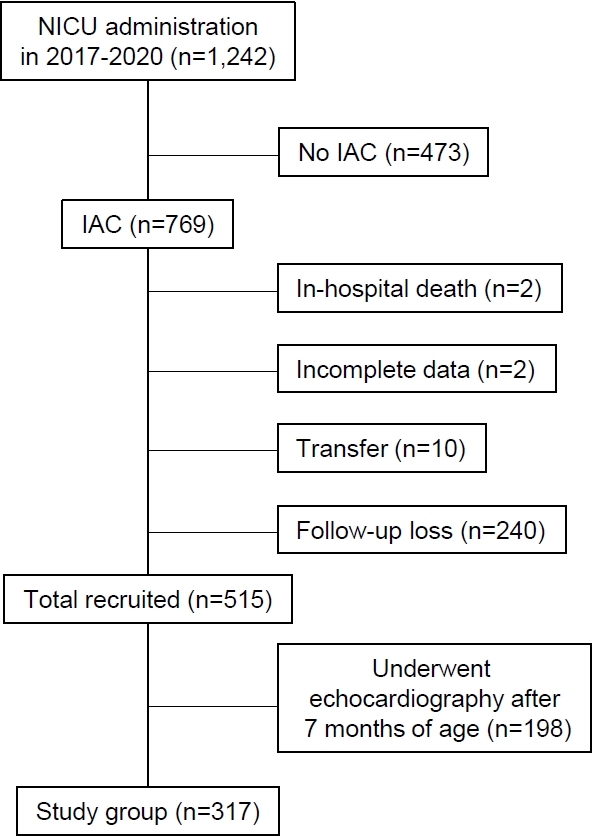
A total of 1,242 infants were admitted to our institutionŌĆÖs NICU between March 2017 and December 2020. Of them, 769 had an interatrial shunt detected on color Doppler echocardiography during the fourth week of life. Infants who died during hospitalization (n=2), were transferred to another hospital (n=10), had incomplete data (n=2), or did not attend follow-up (n=240) were excluded. Among the remaining 515 infants, 198 who did not undergo follow-up examinations before 8 months of age were excluded. Finally, 317 patients were enrolled in this study (Figure 2).
The study included 131 females and 186 males. The patients were admitted to the NICU because of prematurity in 123 (38.8%), respiratory disease in 113 (35.6%), and other manifestations in 81 (25.6%). Of them, 285 did not have IACs, while 32 had IACs. The average defect size and corrected size by birth weight was 3.0┬▒1.0 mm and 1.3┬▒0.7 mm/kg. The duration of hospitalization was 23.3┬▒30.4 days. A total of 100 (31.5%) patients had other hemodynamically significant lesions. In terms of morphological classification, 201 (63.4%) patients had ASD, 97 (30.6%) had PFO, and 19 (6.0%) had multiple holes (Table 1).
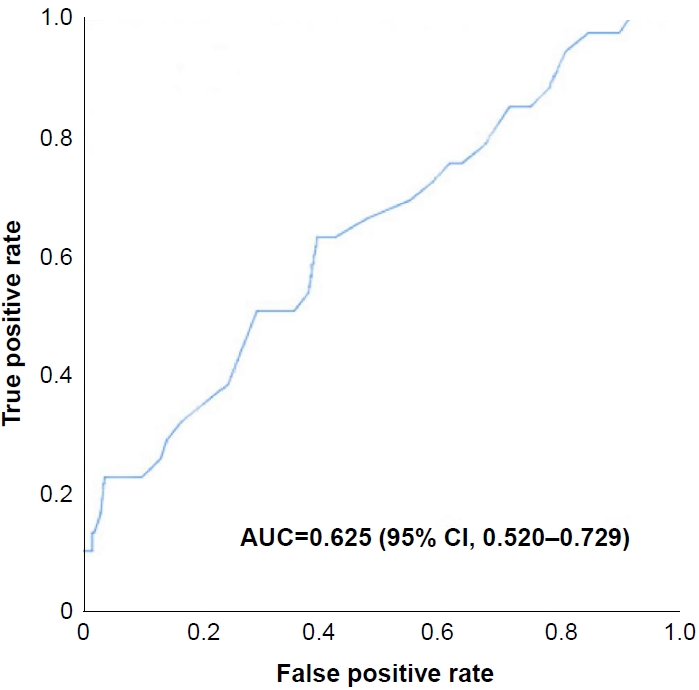
An overall spontaneous closure rate of 89.9% was observed during an average follow-up interval of 5.1┬▒1.1 months. The spontaneous closure rates for each morphological classification were 8.9% for ASD, 10.3% for PFO, and 21.0% for multiple holes. Statistically significant risk factors for the presence of IACs were gestational age, birth weight, defect size, and hospitalization diagnosis (Table 1). When multiple Cox regression analyses were performed to analyze the risk factors of the presence of IACs, a larger initial defect size was estimated associated with a higher risk of IACs at the time of the assessment (Table 2). The optimal cut-off value for predicting spontaneous IAC closure was 3.05 mm, and the area under the ROC curve was 0.625 (95% CI, 0.520 to 0.729) (Figure 3).
Among the 182 preterm infants, the gestational age and birth weight ranged from 23+4 to 36+6 weeks and from 410 to 3,720 g, respectively. The initial defect size was 3.18┬▒1.11 and 3.02┬▒1.01 mm in term and preterm infants, respectively. The defect size corrected by birth weight was significantly different at 0.99┬▒0.36 and 1.65┬▒0.83 mm/kg for term and preterm infants, respectively. There were more concomitant heart lesions in preterm infants (odds ratio, 2.04; 95% CI, 1.241 to 3.372). Preterm infants had a higher closure rate than term infants (odds ratio, 3.34; 95% CI, 1.524 to 7.331). There were no significant differences in gestational age, birth weight, defect size, defect size corrected by birth weight, or concomitant heart lesions in the presence of IACs.
This study investigated the natural course of IACs, which are frequently encountered in NICUs, and the appropriate timing of their follow-up. Many variables play a role in the diagnosis of ASD when echocardiography is performed in newborns. Blood flow through small atrial communication is determined by defect size and relative atrial pressure, which relate to left and right ventricular compliance [6]. If the evaluation is performed at an early age, the pulmonary vascular pressure remains high; therefore, the shunt may not be visible. However, after follow-up, a shunt may be observed. If chamber enlargement due to other shunt lesions or pulmonary pressure hypertension exists, it can be assumed that natural occlusion can be better achieved during follow-up because the size is larger than that of the actual defect. As mentioned above, the presence or absence of a defect may vary depending on a patientŌĆÖs hemodynamic status. However, in this study, the probability of spontaneous closure was not higher among patients with concomitant heart disease. Further research is needed to determine the relationship between hemodynamic status and spontaneous IAC closure.
The initial IAC diameter was the main predictor of spontaneous closure. Hanslik et al. [7], in a study of 200 consecutive patients (median age at presentation, 5 months; median follow-up, 4 to 5 years), reported spontaneous closure in 56% of patients with an initial defect size of 4 to 5 mm, 30% in those with 6 to 7 mm defects, and 12% in those with 8 to 10 mm defects. An Iranian study concluded that ASDs <6 mm typically close spontaneously, while those 6 to 9 mm may regress in infants and children. When >1 cm, the probability of spontaneous closure is poor [8,9]. Radzik et al. [10] suggested that infants born with an ASD <3 mm may not have a cardiac malformation and therefore do not require follow-up examinations because spontaneous closure occurs in all cases. In contrast, for ASD Ōēź8 mm, regular echocardiography is required because percutaneous or surgical interventional occlusion may be required [10]. In this study, defect size was a significant predictor of spontaneous IAC closure, and it was investigated in cases of Ōēż3 mm, consistent with previous studies. In the case of premature infants and those with low birth weights, the relative defect size could affect spontaneous occlusion. However, defect size corrected by birth weight was not a predictor of spontaneous occlusion.
Several mechanisms have been proposed to explain spontaneous closure, such as downward growth of the septum secundum, formation of thrombotic plugs, formation of septal aneurysms, and fusion of valve-like openings [11-14]. However, many of these hypotheses are questionable regarding whether it is possible to examine atrial septal development. Anderson et al. [15] explained septal development as follows: the flap valve, which forms the floor of the fossa and is free to open and close when the fossa is patent, is an exemplar of the shelf-like partition that we define as a true septal structure. The anteroinferior muscular buttress, which anchors the flap valve to the fibrous atrioventricular septal structures, is also a true septum that can be removed without communication with the extracardiac space. The septum is created by deep infolding between the walls of the superior caval and the right pulmonary veins [15]. Therefore, further studies are needed to identify factors related to spontaneous closure of the IAC.
The present study had several limitations. The retrospective identification of study patients introduced the potential for selection bias. For example, patients who cannot be followed up may be less ill or transferred due to severe illness. However, it has the advantage of minimizing observation bias by monitoring patients according to a standardized protocol and storing echocardiographic data in the database during examination. This study did not examine whether other echocardiographic measurements were associated with the natural course of IAC because such data were not collected in a standardized manner. And finally, long-term follow-up of the study participants was lacking.
In conclusion, other than large defect size, no factors delayed spontaneous closure. When defects in the oval fossa measure Ōēż3 mm, most patients experience spontaneous closure by 7 months of age (term infants) or 7 months of corrected age (preterm infants). These results suggest that follow-up may not be necessary because most infants with an ASD of <3 mm experience spontaneous closure, and that follow-up to 7 months of age in term infants and corrected age of 7 months in preterm infants is reasonable. Moreover, defects <3 mm probably do not constitute a cardiac malformation owing to their natural evolution.
ARTICLE INFORMATION
Ethical statement
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Institutional Review Board of Soonchunhyang University Bucheon Hospital (SCHBC 2022-08-022). Written informed consent by the patient was waived due to a retrospective nature of our study.
Figure┬Ā1.
Morphological subtypes of secundum atrial septal defect (ASD) in the center of the oval fossa. (A) The ASD is located within the true septum. The defect margin is clear cut, and the shunt flow and interatrial septum (IAS) are almost vertical. (B) The patent foramen ovale is a tunnel-like passageway between the free edge of the overlapping ovale fossa valve and its muscular rim. This type includes cases in which adhesion does not occur or a shunt occurs due to a stretched secondary septum. Shunt flow and IAS angle is less than 90┬░. (C) If there are more than two holes, the patient is classified as having multiple holes. Arrow points left-to-right flow imaging by color Doppler. Abbreviations: LA, left atrium; RA, right atrium.
Figure┬Ā2.
Enrollment of the study group. NICU, neonatal intensive care unit; IAC, interatrial communication.
Figure┬Ā3.
Receiver operating characteristic curve showing that the optimal cut-off value for predicting spontaneous interatrial communication closure was 3.05 mm. Abbreviations: AUC, area under the curve; CI, confidence interval.
Table┬Ā1.
Clinical Profiles of Newborns with Interatrial Communications
| Clinical profiles | Total (317 cases) | Absence of IACs (285 cases) | Presence of IACs (32 cases) | |
|---|---|---|---|---|
| Gestational age (wk)* | 35.5┬▒3.6 | 35.3┬▒3.6 | 37.3┬▒3.0 | |
| Birth weight (g)* | 2,552.2┬▒819.9 | 2,507.2┬▒810.1 | 2,952.8┬▒810.6 | |
| Age at diagnosis (d) | 4.7┬▒3.6 | 5.0┬▒3.3 | 4.1┬▒3.1 | |
| Male sex | 186 (58.6) | 170 (59.6) | 16 (50.0) | |
| Diagnosis of hospitalization* | ||||
| Preterm | 123 (38.8) | 117 (41.0) | 6 (18.7) | |
| Respiratory disease | 113 (35.6) | 97 (34.0) | 16 (50.0) | |
| Others | 81 (25.6) | 71 (25.0) | 10 (31.3) | |
| Defect size (mm)* | 3.0┬▒1.0 | 3.0┬▒1.0 | 3.6┬▒1.3 | |
| Corrected size by birth weight (mm/kg) | 1.3┬▒0.7 | 1.3┬▒0.7 | 1.3┬▒0.7 | |
| Duration of hospitalization (d) | 23.3┬▒30.4 | 24.1┬▒31.5 | 16.6┬▒15.7 | |
| Concomitant heart lesions | 100 (31.5) | 91 (31.9) | 9 (28.1) | |
| Patent ductus arteriosus | 87 (27.4) | 80 (28.0) | 7 (21.8) | |
| Patent ductus arteriosus and ventricular septal defect | 5 (1.5) | 4 (1.4) | 1 (3.1) | |
| Patent ductus arteriosus and persistent pulmonary hypertension | 4 (1.2) | 4 (1.4) | 0 | |
| Ventricular septal defect | 4 (1.2) | 3 (1.0) | 1 (3.1) | |
| Morphology of the defect | ||||
| Atrial septal defect | 201 (63.4) | 183 (64.2) | 18 (56.2) | |
| Patent foramen ovale | 97 (30.6) | 87 (30.5) | 10 (31.2) | |
| Multiple holes | 19 (6.0) | 15 (5.3) | 4 (12.6) | |
Table┬Ā2.
Results of a Multiple Cox Regression Analysis of the Risk Factors of Presence of Interatrial Communications
REFERENCES
3. Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: incidental or pathogenic? Stroke 2009;40:2349ŌĆō55.



4. Kasper W, Geibel A, Tiede N, Just H. Patent foramen ovale in patients with haemodynamically significant pulmonary embolism. Lancet 1992;340:561ŌĆō4.


5. Konstantinides S, Geibel A, Kasper W, Olschewski M, Blumel L, Just H. Patent foramen ovale is an important predictor of adverse outcome in patients with major pulmonary embolism. Circulation 1998;97:1946ŌĆō51.


6. Le Gloan L, Legendre A, Iserin L, Ladouceur M. Pathophysiology and natural history of atrial septal defect. J Thorac Dis 2018;10(Suppl 24): S2854ŌĆō63.



7. Hanslik A, Pospisil U, Salzer-Muhar U, Greber-Platzer S, Male C. Predictors of spontaneous closure of isolated secundum atrial septal defect in children: a longitudinal study. Pediatrics 2006;118:1560ŌĆō5.



8. Behjati-Ardakani M, Golshan M, Akhavan-Karbasi S, Hosseini SM, Behjati-Ardakani MA, Sarebanhassanabadi M. The clinical course of patients with atrial septal defects. Iran J Pediatr 2016;26:e4649.



9. Saxena A, Divekar A, Soni NR. Natural history of secundum atrial septal defect revisited in the era of transcatheter closure. Indian Heart J 2005;57:35ŌĆō8.

10. Radzik D, Davignon A, van Doesburg N, Fournier A, Marchand T, Ducharme G. Predictive factors for spontaneous closure of atrial septal defects diagnosed in the first 3 months of life. J Am Coll Cardiol 1993;22:851ŌĆō3.


11. Cayler GG. Spontaneous functional closure of symptomatic atrial septal defects. N Engl J Med 1967;276:65ŌĆō73.


12. Sherman FS, Sahn DJ, Valdes-Cruz LM, Chung KJ, Elias W. Twodimensional Doppler color flow mapping for detecting atrial and ventricular septal defects: studies in an animal model and in the clinical setting. Herz 1987;12:212ŌĆō6.

13. Timmis GC, Gordon S, Reed JO. Spontaneous closure of an atrial septal defect. JAMA 1966;196:17ŌĆō9.









