Hearing Impairments in Preterm Infants: Factors Associated with Discrepancies between Screening and Confirmatory Test Results
Article information
Abstract
Purpose
The aim of the study was to investigate risk factors of hearing impairments in preterm infants and analyze factors associated with discrepancies between neonatal hearing screening (NHS) and confirmatory test results.
Methods
We analyzed the medical records of 352 preterm infants born at 23 to 32 weeks’ gestational age (GA) who underwent both automated auditory brainstem response (aABR) and confirmatory ABR (cABR).
Results
Mean GA, mean birth weight, the incidence of small for GA and cesarean section birth were significantly different between the pass and refer groups on aABR and the normal and abnormal groups of cABR. On univariate analysis, bronchopulmonary dysplasia (odds ratio [OR], 2.74; 95% confidence interval [CI], 1.00 to 7.48), intraventricular hemorrhage (OR, 7.02; 95% CI, 1.59 to 31.05), and use of furosemide (OR, 3.84; 95% CI, 1.38 to 10.73) were the factors related to refer results on aABR. Periventricular leukomalacia (PVL; OR, 4.00; 95% CI, 1.39 to 11.52) and use of vancomycin (OR, 2.86; 95% CI, 1.22 to 6.73) were associated with abnormal cABR. Twenty-five (7.9%) infants had discrepant aABR and cABR results, particularly males and those in whom vancomycin was used.
Conclusion
PVL and use of vancomycin were confirmed as independent risk factors for hearing loss in infants born at less than 32 weeks’ GA. Also, discrepancies between the screening and confirmatory test may occur, especially among male infants and those in whom vancomycin was used. The hearing of infants must be assessed more carefully in such groups regardless of NHS results.
INTRODUCTION
Hearing impairment (or hearing loss [HL]) is among the major neurodevelopmental deficits associated with preterm birth [1-3]. The estimated incidence of bilateral permanent hearing loss (PHL) is 1 to 3 per 1,000 live births in the well infant population, and premature babies are at higher risk of hearing problems [4]. Despite the recent remarkable increase in survival of extremely preterm infants, the incidence of severe sensorineural hearing loss (SNHL) remained unchanged for over 30 years [5]. Gestational age (GA) and birth weight are inversely related to hearing problems [1,2,6].
Without appropriate stimuli reaching the auditory center of the brain, infants cannot achieve normal linguistic and socialemotional development and later tend to experience psychological and mental disorders [2,7]. Accordingly, it is critical that severe HL be detected in infants early on, especially premature infants. Although various screening protocols exist across countries and medical centers, the automated auditory brainstem response (aABR) is now widely used for screening for HL in newborn babies [5,8]. When the results of the screening test are suggestive of PHL, otoacoustic emissions and/or confirmatory auditory brainstem response (cABR) is performed to confirm actual hearing impairments. Both are noninvasive and can easily be performed on neonates and infants [2,4,8]. Among the different screening protocols, ABR is the most objective confirmatory technique [7].
In clinical practice, infants with congenital HL may receive a pass result on the neonatal screening test, meaning that the early detection of hearing impairment fails and timely treatment is not provided [9]. Effort is needed to identify the factors related to discrepancies between screening and confirmatory test results. By achieving the early recognition of infants with HL, we can provide effective treatments for them. The diagnosis should be made within 3 months after birth, as early intervention is needed before 6 months of age [2,7].
The Joint Committee on Infant Hearing reported several risk factors associated with PHL in 2000 and updated the list in 2007 [8]. Many other risk factors have been reported, including ototoxic drugs, prematurity, very low birth weight, a low Apgar score, and prolonged duration of mechanical ventilation [1,4,6,10-15].
In this study we investigated the risk factors of hearing impairment in preterm infants born at less than 32 weeks’ GA and analyzed the factors that cause discrepancies between neonatal hearing screening (NHS) and confirmatory test results.
MATERIALS AND METHODS
The study was a retrospective single-center cohort study conducted at the neonatal intensive care unit (NICU) of Severance Children’s Hospital, Yonsei University College of Medicine, Seoul, Korea. Of the 352 preterm infants born at 23 to 32 weeks’ GA between August 2008 and December 2017, five infants with genetic or major structural anomalies such as skeletal dysplasia, coarctation of the aorta, severe congenital ventriculomegaly, cleft palate, and Down syndrome were excluded. We reviewed the electronic medical records of the remaining 347 infants who underwent both aABR and cABR testing.
As soon as the general condition of infants allowed, NHS testing using the aABR method was performed in the NICU prior to discharge. A reliable response from the infant at a threshold level of 35 dB on the test leads to a pass result; otherwise, a refer result is given. Regardless of the aABR results, all infants also underwent cABR testing performed by a specialized technician from the Department of Otolaryngology. HL was diagnosed when the cABR test result was ≥40 dB.
For analyses of risk factors for hearing impairment in general, information on a variety of factors along with those already known to be associated with hearing impairments like GA at birth, small for gestational age (SGA), and a low Apgar score were collected. SGA was defined as having a birth weight <10th percentile for the given GA. Some complications of prematurity reported by some studies as risk factors of HL in premature babies were chosen for the analysis, including bronchopulmonary dysplasia (BPD), periventricular leukomalacia (PVL), intraventricular hemorrhage (IVH), culture-proven sepsis, retinopathy of prematurity, and patent ductus arteriosus. Prolonged duration of mechanical ventilation and some ototoxic drugs known to make hearing problems were also included.
Discrepancies between the aABR and cABR tests were categorized into two groups: those with a refer result on the aABR test and a normal result on the cABR test (group of nonconcern [GN]); and those with a pass result on the aABR test and abnormal result on the cABR test (group of concern [GC]). With a refer aABR test result in routine clinical settings, the infants are bound to undergo the cABR test within 3 months to ascertain whether there are actual hearing impairments; thus, this type of discrepancy would raise little safety concern in the clinical setting as with GN group. However, for the other group GC in clinical settings, where a pass result on the aABR test means no cABR test required, there is a high probability that the infants might miss the opportunity of detecting HL in time for proper treatments. We focused our analysis only on this latter group.
Data are given as mean±standard deviation. For continuous variables with a normal distribution and homogeneous variance, t-tests were performed to compare two groups. For nominal variables, the chi-square test or Fisher’s exact test was performed. Binary and multiple logistic regression models were used to identify factors significantly associated with abnormal results on the aABR and cABR tests. Odds ratio (OR) and 95% confidence interval (CI) were also calculated. Statistical significance was considered at values of P<0.05. All statistical analyses were performed using SPSS version 25.0 (IBM Co., Armonk, NY, USA).
RESULTS
Of the 347 preterm infants, the mean GA at birth was 29.2±2.0 weeks and mean birth weight was 1,232±367 g. A total of 267 (76.9%) neonates were born by cesarean section, 52 (15.0%) were SGA, and 189 (54.5%) were male.
On the aABR method, 317 (91.4%) neonates showed a pass result and other 30 (8.6%) neonates had a refer result (Table 1). GA (29.3±2.0 weeks vs. 28.1±2.0 weeks, P=0.002), birth weight (1,261±358 g vs. 933±328 g, P<0.001), and 1-minute Apgar score (3.9±1.5 vs. 2.9±1.2, P=0.001) were significantly lower and a higher incidence of SGA infants (12.0% vs. 46.7%, P<0.001) and babies born by cesarean section (75.6% vs. 93.3%, P=0.037) were in the refer group. On the cABR test, a total of 41 (11.8%) neonates showed abnormal response and were diagnosed with an HL. As in the aABR group, GA (29.3±1.9 weeks vs. 28.4±2.3 weeks, P=0.011) and birth weight (1,259±364 g vs. 1,039±335 g, P<0.001) were lower in the abnormal cABR group. A higher incidence of infants born with SGA and by cesarean section was also reported in the abnormal group.
Risk factors affecting hearing impairments ware shown in Table 2. By binary logistic regression modelling, moderate or severe BPD (OR, 2.74; 95% CI, 1.002 to 7.48), grade 3 or 4 IVH (OR, 7.02; 95% CI, 1.59 to 31.05), and use of furosemide for more than 14 days (OR, 3.84; 95% CI, 1.38 to 10.73) were significantly associated with abnormal aABR test results. In multiple logistic regression modelling with the adjustment for bias by prematurity using GA, grade 3 or 4 IVH (OR, 4.65; 95% CI, 1.13 to 19.17), and use of furosemide (OR, 3.91; 95% CI, 1.72 to 8.87) were still associated with a refer result on the aABR test. PVL (OR, 4.00; 95% CI, 1.39 to 11.52) and use of vancomycin for more than 5 days (OR, 2.86; 95% CI, 1.22 to 6.73) were associated with an abnormal cABR test on binary logistic regression. After the adjustment for GA, they remained significant risk factors for an abnormal cABR (OR, 4.03; 95% CI, 1.42 to 11.39 for PVL) (OR, 2.34; 95% CI, 1.02 to 5.38 for use of vancomycin).
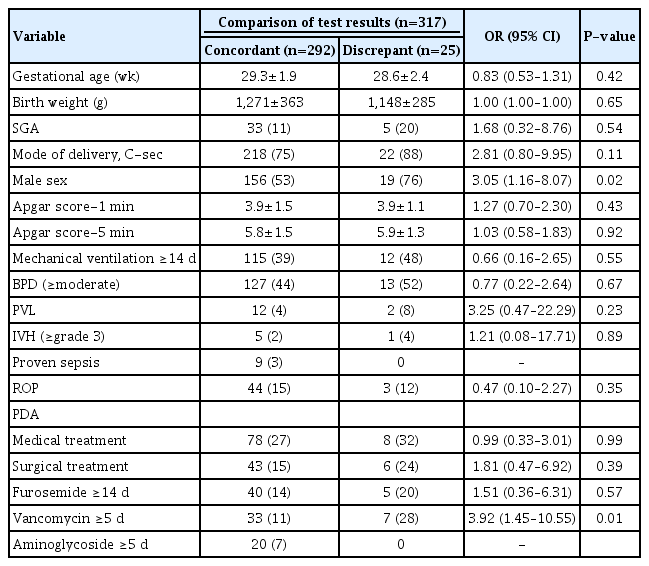
Factors related to an abnormal cABR result among patients with a normal aABR result are listed below (Table 3). Twenty-five (7.9%) infants had inconsistent aABR versus cABR test results, meaning that they passed the aABR test but had abnormal cABR test results. Infants who were male (OR, 3.05; 95% CI, 1.16 to 8.07) and had a history of vancomycin use for more than 5 days (OR, 3.92; 95% CI, 1.45 to 10.55) were at significantly higher risk of having inconsistent results on the multiple logistic regression model with the adjustment for confounding factors like GA or birth weight.
DISCUSSION
Hearing impairment is among the most important consequences of preterm birth. The NHS test is considered an effective method for the early detection of hearing impairments in infants; therefore, it should be of priority in neonatal care [16,17]. The goal of early detection and intervention for HL is to maximize linguistic competence and literacy development in children.
Routine NHS testing of all neonates in Korea began in 2007. Since then, the implementation of NHS programs for newborns has continued to grow. However, the sensitivity and accuracy of such NHS tests remain issues. The interpersonal and interinstitutional variance of the test are also concerning, especially among preterm infants. To our knowledge, this is the first study to analyze risk factors for the discrepant results between aABR and cABR testing. Here we show the results of abnormal hearing screening tests in preterm infants in a single center in Korea over a 10-year period.
The pathophysiology of HL in preterm infants is complex. Along with prematurity, SGA, and a low Apgar score, HL is commonly associated with multiple risk factors that can influence hearing in an additive fashion. A family history of hearing deficits and genetic diseases that can affect the auditory system reportedly influence the degree of impairment. Thus, we excluded infants with any genetic diseases from the analysis of the discrepancies.
Other causes particularly important to HL in premature infants include ototoxic drugs such as aminoglycosides, vancomycin, and loop diuretics [8,10,11,18]. The ototoxicity of aminoglycoside depends on treatment duration, serum peak and trough concentrations, concomitant diseases, and the simultaneous administration of loop diuretics and/or vancomycin. Loop diuretics lead initially to reversible HL by blocking ion transport within the stria vascularis in the cochlea [19]. In this study, the use of loop diuretics was among the significant risk factors for an abnormal aABR result but not an abnormal cABR result. This might be explained by the reversibility of HL induced by the use of loop diuretics. If indeed there was enough time between the refer aABR result and the normal cABR result, the ototoxic damage done by loop diuretics may have been resolved by its discontinuation prior to performance of the cABR test.
Another risk factor for HL is exposure to the constant background noise generated by contemporary life-support equipment in the NICU [20]. Robertson et al. [5] showed that mechanical ventilation and prolonged oxygen supplementation were associated with a high prevalence of PHL in extremely premature infants. Hille et al. [21] similarly presented that assisted ventilation for ≥5 days is an independent risk factor for HL. However, in this study, mechanical ventilation lasting more than 14 days did not have an impact on the presence of HL. It is possible that the set point of 14 days was problematic, as only a few days of mechanical ventilation can contribute to hearing impairments as previously reported [21].
Infants with BPD invariably require prolonged periods of mechanical ventilation with an endotracheal tube, resulting in edema of the soft tissues around the Eustachian tube. As suggested previously [22], Eustachian tube dysfunction may exist in high-grade BPD patients, which would predispose them to effusions into the middle ears and, hence, hearing problems. In this study, infants with BPD were at a significantly higher risk of abnormal aABR results (OR, 2.74; 95% CI, 1.002 to 7.48; P<0.05). However, the cABR test results revealed no differences between the normal and abnormal groups. The possible explanations for this discrepancy include recovery from soft tissue swelling of the airway and Eustachian tube maturation, among others. This hypothesis can also be helpful in the analysis of conductive HL in BPD patients.
PVL, a white-matter injury affecting the brains of premature infants, is commonly associated with spastic diplegia, seizures, developmental delay, and visual and hearing impairments [23,24] and results from hypoxic ischemic injury with or without concomitant infection. PVL is characterized by white-matter necrotic lesions, hypomyelination, microglial activation, astrogliosis, and neuronal death. White-matter abnormalities of the auditory neural pathway can cause SNHL [25]. Similarly, PVL was significantly associated with hearing impairments on the cABR test in this study. PVL could be a factor contributing to the discrepancy noted between the aABR and cABR test results because its symptoms usually appear gradually.
The results of the aABR and cABR tests were analyzed by GA and birth weight. Both are widely known factors associated with HL in preterm infants [1,26]. Abnormal results on the aABR and cABR tests tended to occur in infants with a lower GA and lower birth weight. Consistency was noted between the results of the aABR test and the final diagnosis of HL on the cABR test for 10/78 (12.8%) infants born at less than 28 weeks’ GA and 15/239 (6.3%) of infants born at 28 to 32 weeks’ gestation. We can expect that as GA at birth increases, the consistency between the aABR and cABR results will be better maintained.
We may fail to identify some patients with HL because of the imperfect nature of the NHS test. Since the risk of HL in preterm infants is greater than that in the normal group, despite the normal result of the screening test, we must carefully check for the presence of HL. In this study, male infants and those in whom vancomycin was used were significant risk factors for a discrepancy between the NHS and confirmatory test results. There can be some sex-based differences in several aspects of the brain; with regard to the auditory system, men show longer delays on ABR tests than women, mainly because of the slightly longer length of the male cochlea [27]. No studies have focused on sexual differences in infants with congenital HL, and further studies with many more patients are needed to identify the exact correlation between sex and hearing impairments.
Although debate persists, vancomycin is sometimes said to be an ototoxic drug [10]. Vestibular and/or cochlear damage associated with tinnitus and SNHL have been reported in humans after the use of vancomycin. Some studies have reported that the concomitant use of aminoglycosides and vancomycin can cause hearing problems; however, in this study, no infants were administered both drugs. The use of vancomycin was a risk factor significantly associated with an abnormal cABR test result and could cause the discrepancy in aABR and cABR test results, probably by inducing a delayed HL. Also, despite the uncertainty of its ototoxic effects in the normal population, vancomycin may have a greater effect on hearing impairments in some relatively vulnerable groups, such as preterm infants. Furthermore, a relatively short treatment duration of 5 days or the concomitant use of other ototoxic drugs like furosemide may have confounded the results. More studies are needed to confirm the possibility and mechanisms of ototoxicity with vancomycin use.
This study has several limitations. First, it was not a prospective cohort study; thus, some confounding factors could have affected the results. Second, the aABR and cABR tests were conducted at different times. Third, the sensitivities and specificities differ between the aABR and cABR; thus, some bias in risk factors may be unavoidable. Finally, the relatively small number of infants with abnormal hearing could have caused some errors in the statistical analysis. Further studies with a greater sample of preterm infants should be conducted.
In conclusion, this study confirmed that PVL and use of vancomycin for more than 5 days as independent risk factors for HL despite the adjustment for GA in infants born at less than 32 weeks’ GA. Careful consideration of the use of vancomycin and a timely examination for HL are needed. We also found that, especially in male infants and those in whom vancomycin was used, a discrepancy existed between the screening and confirmatory test results. It is important to carefully assess highrisk babies for false-negative results on the NHS test. Further studies on the risk factors of hearing impairments in premature babies are needed to ensure their normal future development.
Notes
Ethical statement
This study was reviewed and approved by the Institutional Review Board of Severance Children’s Hospital (4-2020-0364), and was conducted in accordance with the Declaration of Helsinki. The informed consents were waived since the study design was retrospective chart review in nature.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conception or design: J.H.H., J.E.S., H.S.E.
Acquisition, analysis,or interpretation of data: J.H.H., M.S.P.
Drafting the work or revising: S.M.L., K.I.P.
Final approval of the manuscript: S.M.L., M.S.P.
Acknowledgements
None