Association between Serum Hyponatremia and Severity of Respiratory Symptoms in Infants with Respiratory Syncytial Virus Infection
Article information
Abstract
Purpose
Association between hyponatremia and the severity of respiratory symptoms in infants with respiratory syncytial virus (RSV) infection has not yet been studied. This study aimed to compare respiratory symptoms, assessed using the Pediatric Respiratory Score (PRS), in infants with RSV infection, with or without hyponatremia.
Methods
RSV-positive patients aged <12 months who were admitted with respiratory symptoms within 7 days of onset at Jeonbuk National University Children’s Hospital from January 2016 to December 2019 were retrospectively analyzed. Each patient was categorized into those with or without hyponatremia (serum sodium concentration of <136 mmol/L). Clinical findings included PRS on the day of admission.
Results
The mean±standard deviation age of the 125 patients included in the study was 2.7±3.3 months, and, 20 patients (16.0%) showed hyponatremia. Infants with RSV infection and hyponatremia had lower birth weights, longer hospital stays, and higher blood urea nitrogen level. The C-reactive protein level was significantly higher in the hyponatremic infants, who had higher PRSs. The non-hyponatremia group had more normal PRSs than the hyponatremia group, which had more severe PRSs. After adjustment for age at admission, blood urea nitrogen level (OR, 1.218; 95% CI, 1.023 to 1.451; P<0.05), and PRS grade (OR, 2.885; 95% CI, 1.158 to 7.187; P<0.05) were identified as independent risk factors.
Conclusion
Hyponatremia was strongly associated with respiratory severity in infants with RSV. Therefore, infants admitted with RSV infection who show higher PRS grade need to be evaluated and treated for hyponatremia.
INTRODUCTION
Respiratory syncytial virus (RSV) is one of the most important causes of respiratory virus infection in infants. Patients with RSV infection show various respiratory symptoms, including lower respiratory tract infection. In addition, they can present with non-respiratory related symptoms such as cardiovascular failure, cardiac arrhythmias, central apnea, seizures, hyponatremia, and hepatitis [1]. Among these symp toms, hyponatremia is closely re lated to disease severity in patients with the infection. While hyponatremia was observed in 33% of young children requiring intensive care unit (ICU) admission associated with RSV infection, only 0.6% showed hyponatremia among those with mild symp toms [2,3]. Other studies have not correlated RSV infection with hyponatremia alone [4]. In other words, hyponatremia is related to RSV infection severity.
Seattle Children’s Hospital developed a scoring system, the Pediatric Respiratory Score (PRS) (updated in 2018), which is used to assess asthma severity in all pediatric patients, including infants. The variables included in this scoring system are respiratory rate, chest retraction, dyspnea, and auscultation findings [5]. Respiratory symptoms can be evaluated numerically using this standardized system in infants and young children with respiratory infections.
Any association between hyponatremia and the severity of the respiratory symptoms in infants with RSV infection has not yet been studied. This study aimed to compare the respiratory symptoms, as assessed using the PRS, between infants with RSV infection with and without hyponatremia at admission.
MATERIALS AND METHODS
1. Patients
This study was a retrospective analysis of RSV-positive patients aged <12 months who were admitted with respiratory symptoms within 7 days of onset at Jeonbuk National University Children’s Hospital (JNUH) over 4 years (January 2016 to December 2019). This study was approved by the Institutional Review Board of JNUH (IRB No. 2020-03-043). All the patients were confirmed to have RSV infection on the basis of a nasopharyngeal swab using a respiratory polymerase chain reaction (PCR) panel, which included RSV, influenza virus, parainfluenza virus, coronavirus, rhinovirus, adenovirus, human metapneumovirus, bocavirus, and enterovirus. Any patients who tested positive for a virus other than RSV were excluded from the study. Patients whose laboratory results were missing or showed hemolysis were also excluded. Patients who had taken any form of diuretics or steroids that could affect the serum sodium level or had underlying diseases such as brain damage, heart anomaly, renal disease, or thyroid disease were also excluded from the study.
2. Clinical and laboratory data
Each patient’s demographic factors, clinical findings, and laboratory results were collected by reviewing admission and progression notes through the electronic medical record system. The demographic factors included age, sex, gestational age, and birth weight. The complete blood count, liver function test results, and levels of serum electrolytes, blood urea nitrogen (BUN), creatinine, glucose, and C-reactive protein (CRP) at admission were reviewed. Hyponatremia was defined as a serum sodium concentration of <136 mmol/L, and each patient was categorized as having RSV infection either with or without hyponatremia [6]. In addition, patients with serum sodium concentrations between 125 and 129 mmol/L were classified as having moderate hyponatremia, and those with concentrations <125 mmol/L were classified as having severe hyponatremia according to the United States guidelines [7]. Dehydration was defined as a fluid deficit of ≥10% of body weight. Clinical findings included vital signs and PRS on the day of admission. The PRS is comprised of four elements, namely respiratory rate, chest retraction, dyspnea, and auscultation findings. Each element was given 0 to 3 points [5]. Infants aged <2 months with respiratory rates of ≤60, 61 to 69, ≥70 breaths per minute were given 1, 2, and 3 points, respectively. Meanwhile, infants aged 2 to 12 months with respiratory rates of ≤50, 51 to 59, and ≥60 breaths per minute were given 1, 2, and 3 points, respectively. For chest retraction, 1 point was given for subcostal or intercostal retraction; 2 points, if two of subcostal, intercostal, substernal retraction, and nasal flaring were present; and 3 points, if 3 or more of subcostal, intercostal, substernal, suprasternal, supraclavicular retraction, nasal flaring, and head bobbing were present. Scores given for dyspnea were based on the patient’s feeding and activity as follows: 1 point, if any feeding intolerance or agitation was present; 2 points, if two of these symptoms were present; and 3 points, if feeding was impossible or the patient was lethargic. Auscultation points were assigned according to the patient’s breath sounds as follows: 1 point, if wheezing was heard only at the end of expiration; and 2 points, if wheezing was heard throughout the whole expiration. If both inspiratory and expiratory wheezing were heard or if the breath sounds were decreased, 3 points were given. The total points for these four elements were categorized into severity groups as follows: normal (0 to 3 points), mild (4 to 6 points), moderate (7 to 9 points), and severe (10 to 12 points).
3. Statistical analyses
The patients’ demographic characteristics, laboratory findings, and PRS at admission were compared between the infants with and without hyponatremia. For continuous variables, the Mann-Whitney U-test or t-test was performed for two groups according to the distribution or homogeneity of the variables. For categorical variables, the chi-square or Fisher exact test was performed. Logistic regression analysis, with stepwise backward elimination, was performed to confirm the risk factor of hyponatremia at admission in patients with RSV infection. All statistical analyses were performed using SPSS version 25.0 (IBM Co., Armonk, NY, USA). Statistical significance was considered as a P-value of <0.05.
RESULTS
1. Incidence and demographics
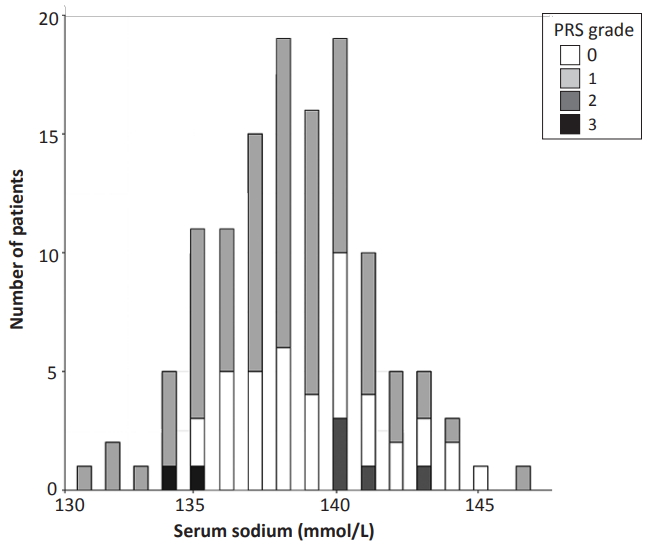
Of the 334 RSV-positive patients aged <12 months who were admitted at JNUH between January 2016 and December 2019, 143 were excluded from this study because they had coinfections based on the respiratory PCR panel. Another 65 patients were excluded because they had inappropriate laboratory results due to missing laboratory or hemolysis data. One patient was excluded because of diuretic medication. None of the patients had brain damage, cardiac anomaly, renal disease, or thyroid disease. The 125 patients included in the study had a mean±SD age of 2.7±3.3 months, of whom 16 (12.8%) were born at <37 weeks of gestational age, while 30 (24.0%) were born at <1 month (<28 days) of gestational age. The mean±SD serum sodium concentration was 138.4±2.8 mmol/L (range, 131 to 147), and 20 (16.0 %) patients had hyponatremia on admission (Table 1, Figure 1).
2. Comparison of patient characteristics and laboratory data
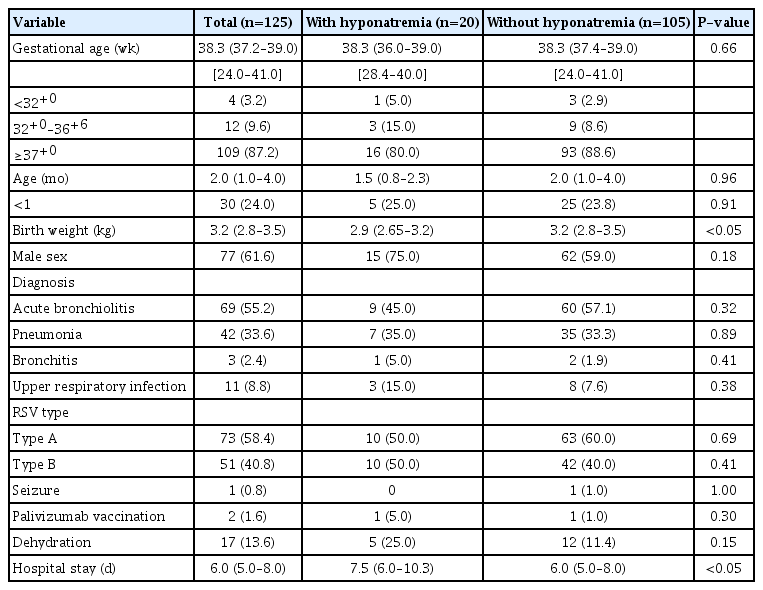
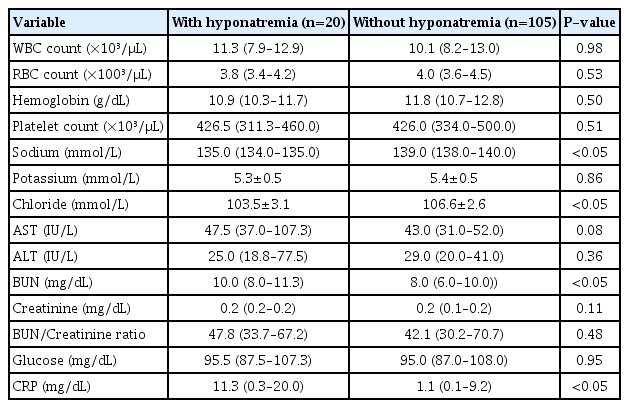
The patients were first categorized into those with or without hyponatremia, and the demographics and clinical characteristics of each group were compared. The patients with RSV infection and hyponatremia had lower birth weights and longer hospital stays. Other factors such as age at admission, gestational age, sex ratio, RSV type, palivizumab vaccination, and dehydration showed no significant differences between the two groups (Table 1). Besides the serum sodium level, serum chloride level was lower and BUN and CRP levels were significantly higher in the patients with hyponatremia. Complete blood count, serum potassium level, liver function tests, and blood glucose level showed no significant differences between the two groups (Table 2). This study did not include any infants with moderate to severe hyponatremia or small for gestational age in both groups.
3. Comparison of PRSs
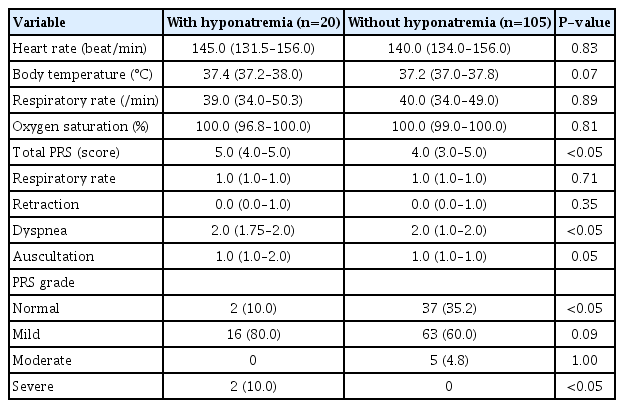
To compare the clinical symptoms of RSV infection between the two groups, vital signs and PRSs at admission were compared. At the time of admission, the vital signs were similar, but the PRS was significantly higher in the patients with hyponatremia. The independent elements of dyspnea within the PRS was significantly higher in those with hyponatremia. The non-hyponatremia group had more normal PRSs than the hyponatremia group (35.2% vs. 10.0%, P<0.05), which had more severe PRSs than the nonhyponatremia group (10.0% vs. 0.0%, P<0.05). The patients with and without hyponatremia showed no significant differences in mild and moderate PRSs (Table 3).
4. Risk factors of hyponatremia among patients with RSV infection
In the univariate logistic regression analysis, birth weight, BUN level, CRP level, and PRS grade were significantly associated with hyponatremia in the patients with RSV infection. After adjusting for age at admission, birth weight (kg; odds ratio [OR], 0.357; 95% confidence interval [CI], 0.145 to 0.881; P<0.05), BUN level (OR, 1.218; 95% CI, 1.023 to 1.451; P<0.05), and PRS grade (OR, 2.885; 95% CI, 1.158 to 7.187; P<0.05) were identified as independent risk factors of hyponatremia in infants with RSV infection (Table 4).
DISCUSSION
A key finding in this study was the association between low serum sodium concentration and more severe respiratory symptoms in infants with RSV infection. Hyponatremia was found to be a common comorbidity in patients with bronchiolitis requiring intensive care and a risk factor for requiring tracheal intubation and a longer ICU stay [8]. Our study results were consistent with these findings in that the length of hospital stay was prolonged and the PRS grade were significantly higher in the patients with hyponatremia. Dyspnea, a PRS element, was significantly worse in the hyponatremic patients at admission because factors such as feeding intolerance and agitation/lethargy were included [9]. Although respiratory rate, chest retraction, and auscultation elements did not show significant differences between the hyponatremia and non-hyponatremia groups, the compilation of each of these elements resulted in a significantly higher total PRS in the hyponatremic group. Hyponatremia was found in 16% of the infants with RSV infection requiring admission in our study, which is comparable with the rates ranging from 13% to 33% in previous similar studies [2,10].
While the association between RSV infection and hyponatremia in children was identified in previous studies, studies solely targeting infants have not been reported to date [2,10]. This may be partly explained by the lack of a comprehensive respiratory scoring system for the infant population. Unlike the Silverman-Anderson score for preterm infants and the Downes score for full-term infants, the PRS developed by Seattle Children’s Hospital includes vital signs and symptoms reflecting the respiratory status of the infant, such as feeding intolerance and activity [11,12]. With the PRS, RSV infection severity in terms of respiratory symptoms could be converted into a measurable index to allow comparison between hyponatremia and non-hyponatremia groups.
The cause of hyponatremia in RSV infection is increased antidiuretic hormone (ADH) secretion, which causes water retention [13,14]. It involves mechanisms such as lung hyperinflation and inflammation. Bronchiolitis in infants causes hyperinflation of the lungs due to air trapping, which increases intrathoracic pressure [15]. This causes increased ADH and renin secretions with secondary hyperaldosteronism, which induces water retention. The inflammatory response to RSV infection is also asso ciated with increased ADH secretion and hyponatremia. Elevat ed CRP level and high fever, which are signs of severe inflammation, were found in patients with RSV bronchiolitis and hyponatremia [10]. Similarly, our study showed higher CRP levels in the patients with than in those without hyponatremia. Studies have also reported that children with empyema and pneumonia, which are severe forms of lower respiratory tract infection, were strongly associated with moderate or severe hyponatremia [4,16]. A possible secondary immune-mediated mechanism such as inflammatory cytokines, rather than viral invasion itself, was also thought to cause hyponatremia in patients with RSV infection in previous studies [17,18].
Although previous studies showed an association between late hyponatremia at 56 days after birth and very low birth weight (VLBW), the present study did not include VLBW infants, and the current studies showing any relationship between hyponatremia and >1,500-g birth weight are lacking. Therefore, we could not explain the association of body weight with hyponatremia, and further study defining this relationship is needed [19,20].
BUN was associated with RSV infection in patients with hyponatremia. High BUN level can be an indication of hypona tremia due to dehydration. However, considering the facts that the sodium electrolyte imbalance due to dehydration may ap pear as both hypernatremia and hyponatremia, and that no association was found between hyponatremia and dehydration in this study, the possibility of hyponatremia caused by dehy dration is unlikely. Meanwhile, BUN level was a significant predictor of hyponatremia in respiratory infection in a large-scale study [4]. The BUN level in a hyponatremic patient can be a guide in assessing the patient’s state; that is, an increase in BUN level can be related to renal failure, and a decrease in BUN level can observed in the syndrome of inappropriate ADH secretion [21]. The relationship between BUN level and hyponatremia is still unclear, and further study is required. We could not analyze the relationship between ADH level and hyponatremia directly because of the retrospective design of our study, and no data on ADH level was included in the routine laboratory testing regimen. In future research, understanding the relationship between PRS and ADH level in infants with RSV infection would help reveal the hyponatremia mechanism.
In addition to the lack of data on ADH level, this study has other limitations. Studies have investigated seizures caused by severe hyponatremia (114 to 123 mmol/L) and brain edema [2,17] in patients with RSV infection. Although this was an important aspect deserving attention, it was beyond the scope of our study, which did not include any patients with moderate or severe hyponatremia (<130 mmol/L). Lastly, although dehydration (≥10%) was checked at admission, the fact that the input volume in each infant was not measured is considered a limitation of this study.
In conclusion, this study has shown that in infants with RSV infection, hyponatremia was strongly associated with respiratory severity. Therefore, when infants with RSV infection are admitted, the treatment plan must include evaluation and treatment of hyponatremia in patients with higher PRS grade.
Notes
Ethical statement
This study was approved by the Institutional Review Board of Jeonbuk National University Hospital (IRB No. 2020-03-043). Informed consent was waived by the board.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conception or design: S.O.Y., H.H.K., J.K.K.
Acquisition, analysis, or interpretation of data: S.O.Y., H.H.K.
Drafting the work or revising: S.O.Y., H.H.K., J.K.K.
Final approval of the manuscript: H.H.K.
Acknowledgements
None