Clinical Application of Near-Infrared Spectroscopy in Neonates
Article information
Abstract
The incidence of cerebral palsy has not decreased despite advances in neonatal care. Preterm infants are at a high risk of cerebral palsy. Moreover, preterm infants might experience permanent neurological sequelae due to injury in the preterm brain. Although the etiology of preterm brain injury is not fully understood, preterm brain injury is strongly associated with abnormal cerebral perfusion and oxygenation. Monitoring systemic blood pressure or arterial oxygen saturation using pulse oximetry is not enough to guarantee proper cerebral perfusion or oxygenation. Early detection of improper cerebral perfusion can prevent irreversible cerebral damage. To decrease brain injury through the early detection of under-perfusion and deoxygenation, other diagnostic modalities are needed. Near-infrared spectroscopy can continuously and noninvasively monitor regional oxygen saturation (rSO2), which reflects the perfusion and oxygenation status of tissues at bedside. Near-infrared spectroscopy represents a balance between tissue oxygen supply and demand. Cerebral rSO2 monitoring has been used most frequently in neonatal cardiac surgery to monitor cerebral oxygenation and prevent hypoxic damage or shock. Recently, cerebral, renal, or splanchnic rSO2 in neonates is frequently monitored. The progression of a disease, brain injury, and death can be prevented by detecting changes in rSO2 values using near-infrared spectroscopy. In this article, the basic principles, usefulness, and applications of near-infrared spectroscopy in neonates are discussed.
INTRODUCTION
Cerebral palsy occurs in 1 in 500 live births. Its incidence has not decreased despite advances in neonatal care [1]. Preterm infants are at a high risk of cerebral palsy. It affects about 10% of very low birth weight preterm infants born in the United States. In addition, preterm infants might experience permanent neurological sequelae due to injury in the preterm brain [2].
Although the etiology of preterm brain injury is not fully understood, it is known that preterm brain injury is strongly associated with abnormal cerebral perfusion and oxygenation [3]. Suboptimal cerebral oxygenation in preterm infants during the first 2 postnatal weeks is associated with poor neurodevelopmental outcome [4]. Although a relationship between low arterial blood pressure and periventricular white matter injury has been reported [5], monitoring systemic blood pressure or arterial oxygen saturation using pulse oximetry (SpO2) is not enough to guarantee proper cerebral perfusion or oxygenation. This is because SpO2 only reflects arterial oxygen saturation (SaO2), and not real oxygen saturation in deep tissues. Improper tissue perfusion and improper tissue oxygen delivery can be indirectly detected based on decreased SaO2, delayed capillary refill time, elevated blood lactate levels, metabolic acidosis, hypotension, or oliguria, according to their severity. However, these methods are non-specific. In addition, by the time caregivers detect these signs of improper tissue perfusion, it would be too late to recover from the tissue damage. Thus, early detection of improper tissue perfusion is needed to prevent irreversible tissue damage.
The brain is an autoregulatory organ. Thus, cerebral blood flow and pressure can be maintained within the normal range by itself, even when there are fluctuations in the systemic blood pressure. However, in severely ill infants and preterm infants with immature cerebral vascular tree, cerebral autoregulation ceases. Therefore, under-perfusion and deoxygenation may occur even if there are only slight fluctuations in the systemic blood pressure, which would result in hypoxic ischemic brain injury [6]. Decreasing brain injury through the early detection of under-perfusion and deoxygenation requires other diagnostic modalities.
Near-infrared spectroscopy (NIRS) is a noninvasive technique that can continuously monitor regional oxygen saturation (rSO2), which reflects the perfusion status and oxygenation status of underlying tissues at the bedside [7]. NIRS can detect improper tissue perfusion early, before irreversible cell or tissue damage occurs. NIRS predominantly measures the rSO2 of the venous blood, which is composed of a mixture of venous (75%), arterial (20%), and capillary (5%) blood [8]. NIRS represents tissue oxygen delivery and consumption. It reflects the balance between tissue oxygen supply and demand. Cerebral rSO2 monitoring has been frequently used in neonatal cardiac surgery to monitor cerebral perfusion and oxygenation and to prevent hypoxic damage or shock. Recently, cerebral, renal, or splanchnic rSO2 in neonates is frequently being monitored. NIRS is already used to monitor cerebral oxygenation in infants with hemodynamically significant ductus arteriosus (HSDA), cerebral oxygenation in infants with perioperative status of congenital heart disease, and splanchnic oxygenation in infants with necrotizing enterocolitis [9]. In this article, the underlying principles, usefulness, and applications of NIRS in the neonatal intensive care unit will be discussed.
PRINCIPLES AND FUNCTIONS OF NIRS
Near-infrared light (700 to 1,000 nm wavelength) can penetrate living tissues better than visible or ultraviolet light. The light absorption spectra are different between oxygenated and deoxygenated hemoglobin [10]. Oxygenated hemoglobin can absorb more infrared light but less red light than deoxygenated hemoglobin. The NIRS device emits lights of two different wavelengths (730 and 810 nm) from a light emitting diode. These emitted photons can pass through tissues and be absorbed by oxygenated and deoxygenated hemoglobin in living tissues at different ratios according to the oxygenation status of tissues (Figure 1). These photons from the light source can make arc. The non-absorbed fraction of the photons is received by two detectors. The proximal arc detector receives signal from superficial tissues whereas the distal arc detector receives signal from both superficial and deep tissues. The proximal value is subtracted from the distal value. The result represents the oxygen saturation at a depth of 1 to 2 cm in the deep tissue, which represents the rSO2 of the underlying tissue [11]. The rSO2 is obtained using the following formula: rSO2=[oxygenated hemoglobin/(oxygenated hemoglobin+deoxygenated hemoglobin)]. The fractional tissue oxygen extraction (FTOE) is the amount of oxygen extracted from tissues. It reflects the balance between oxygen supply and demand of the underlying tissue. The FTOE is obtained using the following formula: FTOE=[(SaO2–rSO2)/SaO2] [9]. A decrease in rSO2 indicates decreased blood flow, resulting in decreased oxygen delivery to the underlying tissue or increased oxygen extraction by the underlying tissue. Caregivers can check real time rSO2 in order to obtain prompt treatment, to guide interventions and to receive immediate response through specific interventions.
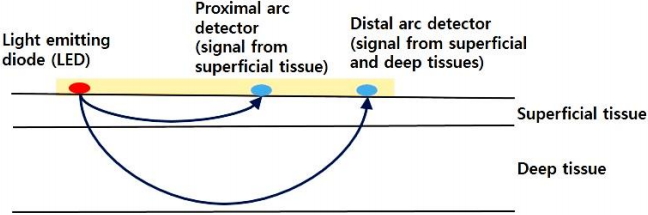
Principles of near-infrared spectroscopy. The nearinfrared spectroscopy device emits light from a light emitting diode. These emitted photons can pass through tissues and become absorbed by oxygenated and deoxygenated hemoglobin in living tissues at different ratios according to the oxygenation status of tissues. These photons from the light source can make arc. The non-absorbed fraction of the photons is received by two detectors. The proximal arc detector receives signal from the superficial tissue whereas the distal arc detector receives signal from both the superficial and deep tissues. The proximal value is subtracted from the distal value and the result represents the oxygen saturation (rSO2) at a depth of 1 to 2 cm in the deep tissue.
CURRENT USE IN NEONATES
In neonates, cerebral, renal, or splanchnic rSO2 is frequently monitored. Cerebral rSO2 is monitored to prevent brain damage in conditions which affect cerebral perfusion and oxygenation. These conditions include hypoxic ischemic encephalopathy, hypotension, apnea and bradycardia, HSDA, ductal closure, and perioperative status of congenital heart disease. Renal or splanchnic rSO2 is monitored to prevent renal or splanchnic underperfusion in infants with necrotizing enterocolitis and those with acute renal failure. Renal or splanchnic rSO2 is also monitored in infants to determine the need for red blood cell transfusion and to monitor response to red blood cell transfusion. There are a number of NIRS devices available for monitoring rSO2 in neonates. These NIRS devices include INVOS 5100 (Somanetics, Troy, MI, USA), Fore-Sight (CAS Medical, Branford, CT, USA), SenSmart X-100 (Nonin Medical, Plymouth, MN, USA), Equanox 7600 (Nonin Medical, Plymouth, MN, USA), and NIRO-200NX (Hamamatsu Photonics, Hamamatsu, Japan), which are commonly used in neonatal intensive care units.
1. Cerebral oxygenation at birth and hypoxic ischemic encephalopathy
Cerebral rSO2 is usually lower than splanchnic or renal rSO2 because the metabolic activity of and demand for oxygen of the brain are higher than those of other living organs [12]. Standard cerebral rSO2 is different according to different studies. It was reported to be 57% to 77% by McCormick et al. [12], 62% to 78% by Lemmers et al. [13], 56% to 76% by Petrova and Mehta [14], and 66% to 83% by McNeill et al. [15]. Generally, the standard neonatal value of cerebral rSO2 is 60% to 80%. Cerebral rSO2 baseline values are affected by altered blood flow due to arteriovenous malformations, superior sagittal sinus, or epidural hemorrhage, by extracranial structures, and by the placement of sensor on the forehead [16,17]. Thus, evaluating the baseline value of cerebral rSO2 is important. After evaluating the baseline cerebral rSO2 value, we need to focus on its trend. Cerebral rSO2 is normally low for several minutes immediately after birth. It then increases [18,19] and becomes stable 7 minutes after birth [20]. Pichler et al. [21] suggested reference ranges for the cerebral rSO2 and FTOE in neonates immediately after birth to prevent cerebral hyper- and hypooxygenation. They reported that the cerebral rSO2 is low at birth. It then increases for several minutes and becomes stable. On the contrary, the cerebral FTOE is high at birth. It then decreases for several minutes and becomes stable (Figure 2) [21]. The cerebral rSO2 was decreased during immediate postnatal 15 minutes in preterm infants who needed FiO2 >0.3 compared to that in preterm infants who needed FiO2 ≤0.3 [11]. This suggests that in some preterm infants, oxygen has to be supplied liberally, not restrictively, to decrease early postnatal hypoxia. On the contrary, Sorensen and Greisen [22] reported that healthy preterm infants have lower FTOE and higher cerebral rSO2 with elevated blood pCO2, meaning higher cerebral oxygenation, compared to term infants. They concluded that prematurity itself is not more prone to cerebral hypoxia than term.

Cerebral regional oxygen saturation (crSO2) and cerebral fractional tissue oxygen extraction (cFTOE) immediately after birth. (A) The 10th, 25th, 50th, 75th, and 90th percentiles of crSO2 during the first 15 minutes after birth in neonates who do not require medical support. The crSO2 is low at birth. It increases for several minutes and becomes stable. (B) The 10th, 25th, 50th, 75th, and 90th percentiles of cFTOE during the first 15 minutes after birth in neonates who do not require medical support. The cFTOE is high at birth. It decreases for several minutes and becomes stable. Adapted from Pichler et al.21), with permission from Elsevier.
Neonates with hypoxic ischemic encephalopathy have increased cerebral rSO2 and decreased cerebral FTOE [23]. This means that cerebral perfusion is increased with improper cerebral autoregulation, while cerebral oxygen consumption is decreased with secondary energy failure during hypoxic ischemic encephalopathy [24]. Among infants with hypoxic ischemic encephalopathy, those with poor neurodevelopmental outcomes have higher initial cerebral rSO2 than those with better neurodevelopmental outcomes [23]. Infants with hypoxic ischemic brain injury identified using brain magnetic resonance imaging have higher initial cerebral rSO2 than those without hypoxic ischemic brain injury [25].
2. Cerebral oxygenation in preterm infants
Preterm infants have different cerebral rSO2 baselines at immediate postnatal stage according to gestational age. Preterm infants have increased cerebral rSO2 and decreased cerebral FTOE compared to term infants at birth. The younger the gestational age, the higher the cerebral rSO2 and the lower the cerebral FTOE [26,27]. Cerebral rSO2 decreases while cerebral FTOE increases according to gestational age at birth, reaching the nadir of rSO2 and the peak of the FTOE at a gestational age between 38 and 39 weeks [28]. Cerebral rSO2 also decreases as preterm infants mature, reaching the nadir between 6 and 8 postnatal weeks [28].
Variations in the cerebral blood flow according to the cerebral perfusion pressure is limited by cerebral autoregulation. Preterm infants are at a high risk of impaired cerebral autoregulation mainly due to immaturity of the cerebral vessels. It has been reported that impaired cerebral autoregulation in preterm infants is highly associated with neonatal death [29]. Caregivers can assess cerebral blood flow and perfusion pressure by measuring cerebral rSO2 using the NIRS in order to quickly identify impaired cerebral autoregulation. Cerebral rSO2 monitoring may help in the early diagnosis of impaired cerebral autoregulation and prevent irreversible brain injury or death [30].
3. Cerebral oxygenation in preterm infants with patent ductus arteriosus
Decreased cerebral oxygenation due to HSDA adversely influences brain growth and neurodevelopmental outcome [31]. Ductus arteriosus requiring surgical ligation was associated with lowest cerebral rSO2 before closure (rScO2 <40%) and brain damage in preterm infants [31]. Preterm infants with HSDA generally have lower cerebral rSO2 but higher cerebral FTOE compared to preterm infants without HSDA [32]. Furthermore, these values are normalized after closure of the HSDA [32]. However, the interpretation of cerebral rSO2 values in relation to ductus arteriosus remains controversial. Schwarz et al. [33] found higher cerebral FTOE (0.43± 0.05 vs. 0.38±0.05, P=0.038) but no difference in cerebral rSO2 (54%±5% vs. 58%±5%, P=0.102) when they compared preterm infants with HSDA to those without HSDA.
There are still controversies about the effect of the closure of ductus arteriosus on cerebral blood flow and cerebral oxygen delivery. Indomethacin decreases cerebral blood flow and cerebral oxygen delivery whereas ibuprofen does not alter cerebral blood flow or cerebral oxygen delivery [34]. Underwood et al. [35] also reported that cerebral rSO2 is decreased whereas somatic rSO2 is increased after the administration of indomethacin. Huning et al. [36] reported that cerebral oxygenation does not change, although cerebral blood flow immediately after ligation of the ductus arteriosus shows a brief increase. On the contrary, Lemmers et al. [37] reported that cerebral rSO2 is decreased immediately after ligation of the ductus arteriosus. Vanderhaegen et al. [38] showed that cerebral rSO2 is increased while cerebral FTOE is decreased during ligation of the ductus arteriosus but returns to baseline following ligation. This suggests that ligation of the ductus arteriosus does not adversely influence cerebral oxygenation.
4. Cerebral oxygenation in infants with perioperative status of congenital heart disease
NIRS has been used most frequently for the perioperative monitoring of cerebral oxygenation during neonatal cardiac surgery to prevent hypoxic damage or shock. The decrease in cerebral rSO2 detected using NIRS during operation to treat congenital heart disease is frequent because cardiopulmonary bypass can cause an abrupt change in cerebral blood flow and oxygenation. Therefore, cerebral rSO2 monitoring is required during cardiac surgery to monitor cerebral oxygenation and to prevent hypoxic damage [39]. Hansen et al. [40] reported that decreased cerebral rSO2 after cardiac surgery might be related to redistribution of blood flow from cerebral circulation to somatic circulation. It has been suggested that increased cerebral vascular resistance and pharmacological afterload reduction can cause redistribution of blood flow from cerebral circulation to somatic circulation, eventually resulting in decreased oxygen delivery to the brain. Low cerebral rSO2 (<56%) detected using NIRS, during the first 48 postoperative hours, is associated with subsequent poor outcome such as death, need for extracorporeal membrane oxygenation, or longer hospitalization in the intensive care unit [41]. Hence, monitoring of cerebral oxygenation is highly recommended to promptly identify patients at risk of poor outcome since poor outcome can be prevented by improving cerebral oxygenation.
5. Splanchnic oxygenation in infants with necrotizing enterocolitis
Splanchnic rSO2 is usually higher than cerebral rSO2 due to higher metabolic activity in the brain. McNeill et al. [15] reported that splanchnic rSO2 is 5% to 15% higher than cerebral rSO2 while Patel et al. [42] reported that the splanchnic rSO2 in preterm infants without necrotizing enterocolitis is 63% to 91%.
Necrotizing enterocolitis is one of the most common gastrointestinal morbidities in preterm infants. The pathogenesis of necrotizing enterocolitis is multifactorial. However, intestinal ischemia is known to precede necrotizing enterocolitis. Preterm infants without necrotizing enterocolitis have higher splanchnic rSO2 during the first week of life compared to preterm infants who develop necrotizing enterocolitis later (77.3% vs. 70.7%, P =0.002) [42]. Preterm infants with splanchnic rSO2 ≤56% are at 11 times increased risk of developing necrotizing enterocolitis compared to preterm infants with splanchnic rSO2 >56% [42]. Low splanchnic rSO2 and high splanchnic FTOE in preterm infants are associated with complicated necrotizing enterocolitis, impending bowel perforation, or death compared to preterm infants with uncomplicated necrotizing enterocolitis [43]. Thus, NIRS monitoring is required to differentiate preterm infants who would subsequently develop complicated necrotizing enterocolitis from those who would not. Splanchnic rSO2 monitoring may contribute to the early diagnosis of necrotizing enterocolitis and prevent bowel perforation or death.
CONCLUSION
NIRS has been widely used in neonatal intensive care units in Korea by the Korean National Health Insurance since 2018. Health insurance coverage for the somatic rSO2 of high risk neonates in the neonatal intensive care unit includes the following: (1) respiratory distress syndrome requiring ventilator care; (2) HSDA or complex heart disease; (3) shock or sepsis; and (4) hypoxic ischemic encephalopathy according to notification no. 2018-184 of the Ministry of Health and Welfare in September 2018.
NIRS can be used for continuous monitoring noninvasively at the bedside without interrupting the routine care of caregivers. Reference ranges of rSO2 and FTOE are different in different infants. They are also different according to postnatal days even in the same infant. Their values are affected by a lot of variables that can affect the oxygen demand and consumption of tissues, such as the metabolic activity of tissues, fever or hypothermia, perfusion status, blood pressure, and level of hemoglobin, especially in preterm infants with anemia. Specifically, splanchnic rSO2 (infra-umbilical) is affected by sensor position, bladder distension, or urinary catheter. Thus, the interpretation of rSO2 values or FTOE values is controversial, and there are no universally accepted normal values. These limitations of NIRS could be the main obstacle to introduce NIRS as a routine bedside monitoring for neonates. However, there are advantages of NIRS monitoring. Caregivers need to focus on the baseline values and trends of changes in these values to prevent the progression of the disease, brain injury, or death. Further studies with a large number of neonates are required to reach a consensus on the uniform interpretation of the values and to develop universally accepted normal values.
Notes
No potential conflict of interest relevant to this article was reported.
