INTRODUCTION
Parkes Weber syndrome (PWS) is a rare congenital vascular anomaly, comprising of capillary, venous, lymphatic, and arteriovenous malformations (AVMs), characterized by hypertrophy of the affected extremity [1]. Capillary malformation (CM) is a slow-flow vascular malformation that forms on the skin as a pink to red macular lesion, otherwise known as a port-wine stain [2]. If this port-wine stain is accompanied by hypertrophy of the affected limb, the differential diagnoses to be considered include PWS, Klippel-Trenaunay syndrome, and Proteus syndrome [3,4]. CM-AVM is a rare condition of atypical CMs associated with fast-flow AVMs and multiple arteriovenous fistulas (AVFs) anywhere in the body [4,5]. Among these CM-AVMs, PWS includes capillary cutaneous malformation, limb hypertrophy, and multiple AVFs of the affected extremity. These AVFs can lead to abnormal bleeding, thrombosis, and heart failure [6,7]. Some cases of PWS and CM-AVMs have the same genetic abnormality due to mutations in the RAS p21 protein activator 1 (RASA1) gene, which affects the development of the vascular system [8,9].
We report here, a case of PWS with the identification of a novel RASA1 splicing mutation.
CASE REPORT
A male infant was admitted to our neonatal intensive care unit for evaluation of severe enlargement and violaceous discoloration of the left upper extremity. His mother was 35 years old and her obstetric history was gravida 3, para 2, living 1. There was no familial history of vascular disease or hemangioma. The patient was born at 39+1 weeks of gestation by repeated Cesarean section. At the time of birth, his Apgar score was 8 at one minute and at 5 minutes. His birth weight was 3,515 g (75th to 90th percentile), height was 49 cm (75th to 90th percentile), and the head circumference was 35.5 cm (above 90th percentile). His vital signs were stable, with a blood pressure of 55/33 mm Hg, heart rate of 152 beats/min, respiratory rate of 27 beats/min, and body temperature of 36.5Ōäā.
On physical examination, the infant had hypertrophy of the left upper extremity. The length of the left arm was 17 cm, 2 cm longer than the right arm, and the middle circumference of the left upper arm was 15 cm, compared to 10 cm of the right arm. The left arm movements occurred freely in the horizontal plane, but there was hardly any mobility in the vertical plane. On the affected area, the port-wine stain was observed and the violaceous color became darker during crying and activity (Figure 1). We felt local warmth with bruit over the left shoulder and upper arm.
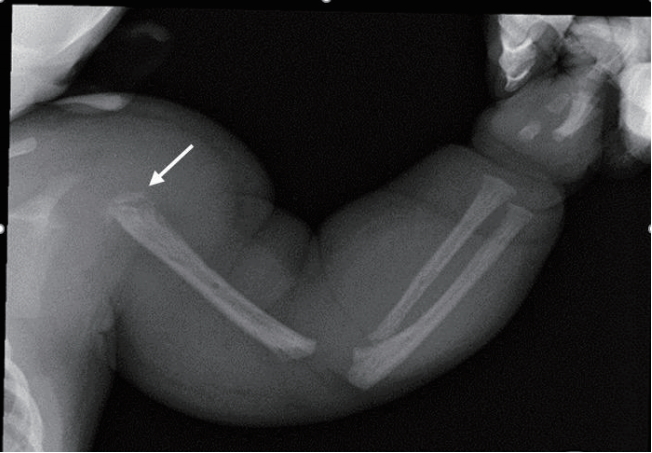
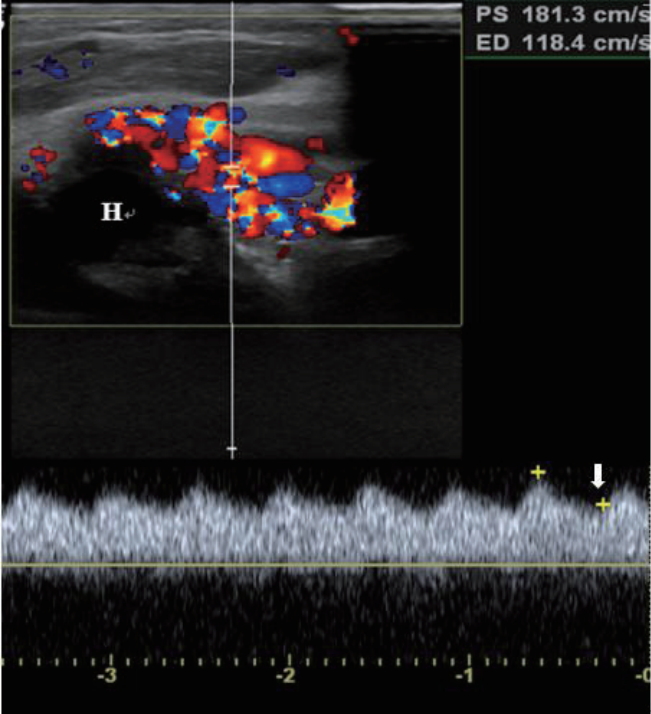
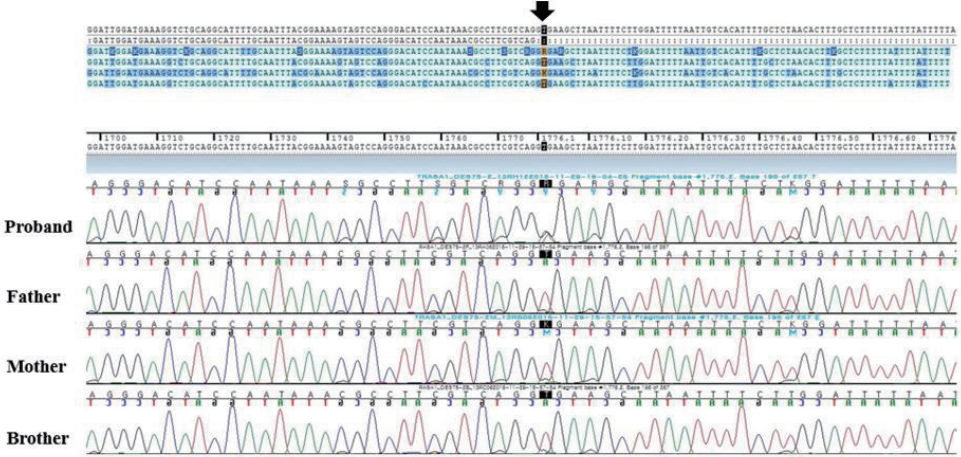
The radiograph of the left arm showed dystrophy of the left humerus with soft tissue swelling (Figure 2). An echocardiogram revealed normal cardiac function and structure except a huge left subclavian artery. On an ultrasound of the left upper arm, high velocity waveform with increased diastolic flow due to an arteriovenous shunt was observed in the upper arm around the humeral head (Figure 3). Magnetic resonance imaging (MRI) and magnetic resonance angiogram (MRA) showed engorged left subclavian and axillary arteries, and their branches included the left circumflex humeral arteries, arteries of the forearm, and muscular branches (Figure 4). Chromosomal analysis revealed a normal male karyotype of 46, XY without structural abnormalities. We performed a molecular genetic study to identify the underlying genetic cause for the limb hypertrophy with a cutaneous CM. Diagnostic exome sequencing test was performed. A novel heterozygous splicing mutation NG_011650.1 (NM_002890.2):c.1776+2T>A was identified in the boundary between exon 13 and intron 13 of the RASA1 gene in the patient. There was no mutation of the gene in his parents and elder brother (Figure 5). Further medical examination included brain ultrasound, abdominal ultrasound, metabolic workup, blood-clotting tests, ophthalmologic examination, and otoacoustic emission, all of which were normal. On the 13th day of hospitalization, he was discharged without hemodynamic changes and diagnosed with PWS. Written informed consent was obtained from the patientŌĆÖs parents for publication of this case report, including images.
DISCUSSION
PWS is a rare congenital vascular anomaly characterized by CM, fast-flow arteriovenous shunt, and hypertrophy of the limb. It was first reported by Frederic Parkes Weber in 1907 [1]. Its exact prevalence is unknown, but approximately 50 patients including three neonates have been reported until 2015 [10,11]. CM is the most common vascular anomaly, which is present at birth and found in 0.3% of the affected infants [2]. It comprises of increased and enlarged capillaries with increased blood flow near the skin, and appears as a pink to red macular lesion, often referred to as a port-wine stain [6]. Unlike the capillary hemangiomas, skin lesions of CM tend to progress with time [4].
CM-AVM is a rare complex vascular disorder, first described by Eerola et al. [5] in 2003. Its clinical features are characterized by atypical multifocal CMs, which are associated with fast-flow AVFs. AVMs and AVFs involve an abnormal connection between arteries and veins that affect blood circulation [6]. They can occur anywhere in the body including cutaneous, subcutaneous, intramuscular, intraosseous, and cerebral regions [3,4]. The clinical symptoms depend on the location and extent of AVMs. These AVFs can lead to severe complications, such as abnormal bleeding, thrombosis, and heart failure [6,11]. Such high-flow AVMs tend to appear during infancy, but some occur later in childhood [3,4]. If there are CMs and limb hypertrophy without defined AVM in an infant, it is difficult to differentiate among PWS, Klippel Trenaunay syndrome (KTS), and Proteus syndrome [8]. KTS is a congenital vascular anomaly, consisting of capillary, venous, and lymphatic malformations, with soft tissue and bony hypertrophy of the affected limb. The cutaneous lesions of KTS are smaller and darker, and the discrepancy in the limblength increases slowly compared to PWS. It is a pure low-flow vascular malformation, while PWS is characterized by highflow AVMs. KTS is associated with the phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) gene [12]. PWS is often confused with KTS until AVMs and the genetic causes are proven. Proteus syndrome is a rare hamartomatous disorder, characterized by various cutaneous and subcutaneous lesions, including vascular malformations, lipomas, and hyperpigmented nevi in addition to hemihypertrophy. Unlike PWS, limb hypertrophy is mild at birth and worsens with age. Proteus syndrome is caused by mutations in the AKT serine/threonine kinase 1 (AKT1) gene [13]. PWS is a kind of CM-AVM, comprising limb hypertrophy with cutaneous capillary stain and multiple AVFs of the extremity. These AVFs can cause local warmth with thrill of the affected limb. In addition, varicose veins and lymphedema may coexist due to venous and lymphatic malformations [4]. In our case, the patient had a pink to red macular lesion and disproportionately severe hypertrophy of the left extremity and local warmth with bruit was felt in the affected limb since birth.
The diagnosis of PWS is usually based on the clinical features described above. Diagnostic imaging is necessary to assess the location and extent of AVMs. Initial evaluation can be performed with Doppler-ultrasound for distinguishing fast-flow AVM from the slow-flow vascular malformation [14]. Further anatomic details are obtained using MRI and MRA, which can reveal musculoskeletal abnormalities, as well as vascular malformations including engorged arteries, veins, capillaries, and flow voids [12,15]. Most cases of PWS are sporadic, although the syndrome might manifest an autosomal dominant inheritance pattern. Some cases of PWS are caused by a mutation in the RASA1 gene [8]. The RASA1 gene mutation was first identified as an aberrant gene in 2008, implicated in the development of PWS [9]. This RASA1 gene is located on chromosome 5 at the position 14.3 and codes for p120-RasGAP, which acts as a negative regulator of RET signaling and affects the G-protein signaling TC21 regulation pathway. The p120-RasGAP has an effect on cell growth, differentiation, and proliferation [16]. Mutations of the RASA1 gene affect CM-AVM and lead to manifestations of PWS, but it is unclear how these mutations affect variable phenotypes [5,8]. According to the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/clinvar/?term=RASA1[gene]), 49 mutations have been reported in the RASA1 gene-related to PWS with a pathogenic variant, and many of these (29/49) are untranslated region mutations as well as missense mutations. So far, there have been no reports of splicing mutations. In the patient presented here, we identified a novel heterozygous splicing mutation in the RASA1 gene.
There is no definite cure for PWS. The goal of treatment for PWS is to improve the patientŌĆÖs quality of life and treatment should be individualized according to the age and clinical features of the patient. Compressive stocking is mainly used as a palliative care for edema and venous ulceration. Invasive treatments such as embolization using a coil, stent-graft implantation, AVM resection, and amputation are considered when complications such as heart failure, venous ulceration, and aneurysm occur [7,11,13]. Recently, sirolimus, a mammalian target of rapamycin (mTOR) inhibitor used for venous and lymphatic malformation, has been tried but its effect has not been proven yet [17].
In the present case, we identified a novel RASA1 heterozygous splicing mutation in a patient with PWS presenting a severely hypertrophied left upper limb with port-wine stain and AVM. To our knowledge, this is the first case of RASA1-related PWS in Korea.