Rapidly Progressive Pericardial Effusion and Cardiac Tamponade in a Term Infant with an Umbilical Venous Catheter: A Case Report
Article information
Abstract
Pericardial effusion (PCE) in neonates has various clinical presentations depending on the amount and speed of fluid accumulation and can cause cardiac tamponade (CT). We report a case of rapidly accumulating PCE and near-fatal CT with an umbilical venous catheter successfully resolved by emergent echo-guided pericardiocentesis in a term infant who had been hospitalized with meconium aspiration syndrome and persistent pulmonary hypertension. This case report suggests that if a patient with an intracardiac umbilical catheter shows sudden cardiopulmonary instability, the possibility of PCE and CT should be considered. Furthermore, if necessary, emergency drainage of the PCE and removal of the umbilical catheter should be immediately performed.
INTRODUCTION
Pericardial effusion (PCE) is a relatively rare medical condition in neonates that can be fatal if cardiac tamponade (CT) occurs. PCE has various clinical presentations depending on the amount of accumulated pericardial fluid and speed of fluid accumulation, from asymptomatic to severe complications, such as CT, which can restrict heart contractility and decrease cardiac output [1].
PCE in neonates can be caused by infections (TORCH, enterovirus), congenital abnormalities, cardiac or pericardial tumor, autoimmune disease, or can be idiopathic or iatrogenic (central venous catheter [CVC]-related complications) [1-3]. The leading cause of PCE in neonates is the presence of an in-dwelling CVCs, both umbilical venous catheter (UVC) and peripherally inserted central catheter (PICC) [4-6]. The median time between placement and onset of PCE was 3 to 4 days. Most patients were preterm infants with a gestational age of <30 weeks, extremely low birth weight (<1,000 g), and had an intracardiac position or misplaced CVC with an ongoing infusion of parenteral nutrition [3]. There have also been reports of central catheter-related PCE and CT cases despite proper placement of the UVC and PICC [4,7]. The mechanisms of central catheter-related PCE and CT remain unknown. A significant number of PCE/CT cases are caused by direct myocardial damage or transmural necrosis due to repeated contact of the catheter tip with the myocardium of the right atrium, and osmotic chemical irritation due to the hyperosmotic infusion of parenteral nutrition [8-13].
Herein, we report a case of rapidly accumulated PCE and near-fatal CT in a term infant with a UVC in situ, who had been hospitalized for meconium aspiration syndrome (MAS) and persistent pulmonary hypertension (PPHN).
CASE REPORT
A female term infant was delivered at a gestational age of 39 weeks and 5 days by cesarean section due to failure of labor progression. She had an APGAR score of 8 at 1 minute and 9 at 5 minutes. The amniotic fluid was meconium-stained during delivery. Two hours after birth, she was transferred to our hospital for the management of tachypnea and chest retraction. Her oxygen saturation level was 92% upon arrival.
Tachypnea, severe chest retraction, and desaturation were observed, and tracheal intubation was performed immediately after arrival. The trachea was suctioned several times using a meconium aspirator and a surfactant was subsequently administered. Her calculated oxygenation index was 20.5 at the initial arterial blood gas analysis. A cardiologist noted a D-shaped left ventricle, tricuspid valve regurgitation, and moderate to severe pulmonary hypertension on echocardiography. A high-frequency oscillatory ventilator with inhaled nitric oxide was used for the treatment of MAS and PPHN in the neonate. A 4 Fr UVC was inserted 3 hours after birth, and total parental nutritional (TPN) support and empirical antibiotics were administered without adjusting the UVC length. The UVC insertion was uneventful, and an umbilical arterial catheter was not inserted. The UVC tip remained inside the cardiac silhouette at the junction of the superior vena cava and right atrium as observed on chest radiography (Figure 1). Echocardiography was used to assess the pulmonary hypertension.

The umbilical venous catheter tip (arrow) remained inside the cardiac silhouette as recorded on chest radiography.
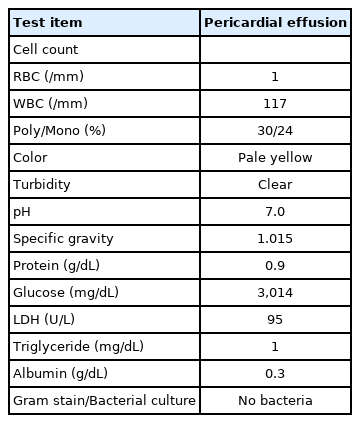
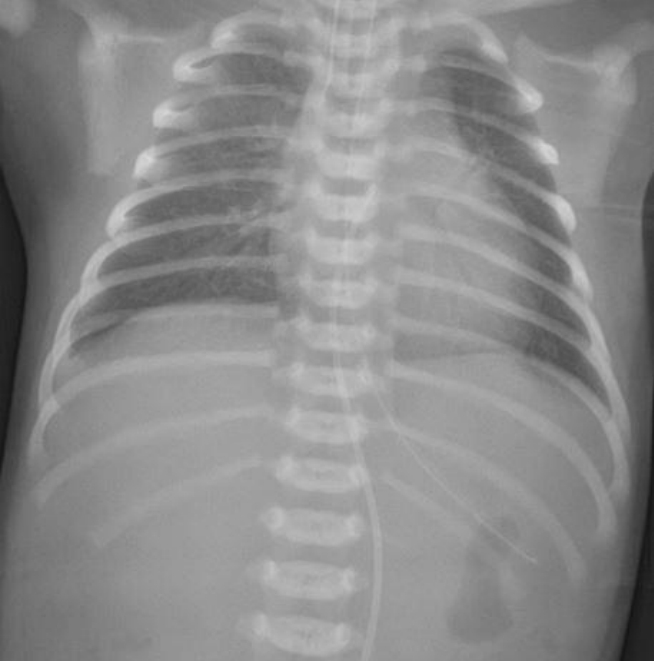
Thirty-two hours after birth (29 hours after UVC insertion), the infant developed sudden hypotension (35/24 mm Hg, mean arterial pressure 28 mm Hg), bradycardia (50 beats/min), and desaturation (SpO2 45%). The sudden cardiopulmonary instability did not improve, even after increasing the doses of dopamine, dobutamine, and inhaled nitric oxide. Chest radiography revealed cardiomegaly with an increase in the cardiothoracic ratio (0.60), when compared to the initial chest radiograph (0.45) (Figure 2). A cardiologist performed a bedside echocardiography, and noted a massive PCE with severe cardiac contractility and output deterioration (Figure 3A). With the diagnosis of PCE and a possible fatal CT, an emergency echocardiography-guided pericardiocentesis with a 24 G intravenous cannula was performed; massive amount of pale yellowish serous fluid was aspirated. The total volume of aspirated PCE was 12 mL (Figure 3B). The UVC tip was located in the left atrium; therefore, the UVC was withdrawn to the diaphragm level. The patient became hemodynamically stable soon after pericardiocentesis, and a follow-up echocardiography confirmed the reduction in PCE and improved cardiac contractility. The serum glucose level at the time of pericardiocentesis was 181 mg/dL.
Chest X-ray after sudden cardiopulmonary instability showed cardiomegaly when compared to the initial chest radiography.
(A) Massive pericardial effusion (arrow) with severe cardiac dysfunction was noted. (B) Pale yellowish serous fluid was aspirated during pericardiocentesis.
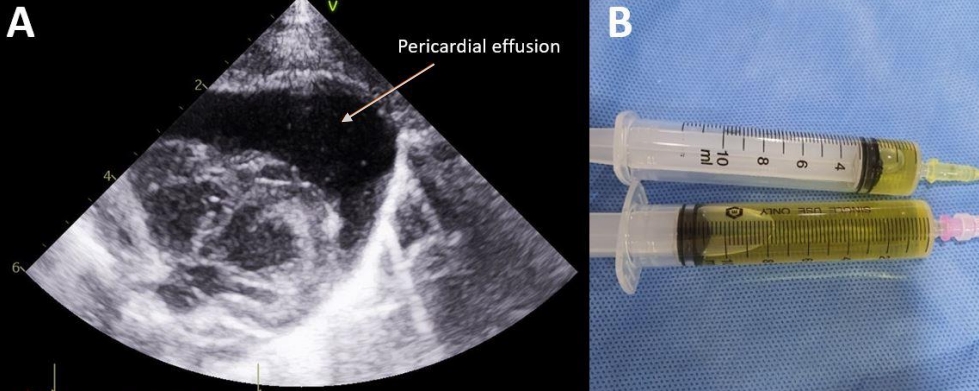
However, 2 hours after the first pericardiocentesis, sudden hypotension, bradycardia, and desaturation recurred. Echocardiography was performed and a recurrence of a massive PCE/CT was noted. A second pericardiocentesis was performed, and an indwelling 4 Fr arrow catheter was inserted (Figure 4). A total of 21 mL PCE was aspirated during the second pericardiocentesis. The UVC was removed after the second recurrence due to a possibility of fluid extravasation. After the removal of the UVC, PCE/CT did not recur. Approximately 800 mOsm/L of TPN fluid containing 10% dextrose and 1.5 g/kg protein was administrated at a rate of 9.2 cc/hr at the time of both PCE and CT occurrences. Biochemical analysis and bacterial culture of the PCE were performed using the first aspirate. The aspirated fluid was biochemically consistent with the TPN fluid, as indicated by the high glucose content (Table 1). No bacteria were identified in the PCE fluid culture.

(A) Recurrence of massive pericardial effusion was noted 2 hours after first pericardiocentesis. (B) An indwelling catheter (arrow) was inserted.
The MAS and PPHN improved, and the patient was extubated on day 3; The in-dwelling pericardial catheter was removed on 4. The total volume of PCE fluid drained through the in-dwelling catheter was 4 mL. She was discharged after 8 days at 40 weeks and 5 days of gestation.
DISCUSSION
The leading cause of PCE in neonates is CVC-related conditions, including in-dwelling PICC and UVC. The risk factors associated with PCE are lower gestational age, lower birth weight, intracardiac position or misplaced CVC, and parenteral nutrition infusion [3-6,9,10]. The catheter tip should not be placed in the right atrium, and the neonate’s movements should be minimized to prevent the migration of the catheter into the right atrium. Some cases of PCE have also been reported in patients with properly placed CVCs that may have been caused by catheter migration. Hence, regularly verifying the correct position of the catheter tip is recommended [7,8,11-13].
Warren et al. [4] reported five cases of PCE/CT in neonates with CVCs that were diagnosed during autopsy. They reported CVC-related PCE/CT cases with appropriate tip positioning and hypothesized that PCE/CT is likely associated with the permeation of hyperosmotic TPN fluid through the right atrial wall without myocardial damage. The microscopic findings of the study included interstitial edema and dilated microvascular channels of the soft tissue. The hyperosmolarity of the TPN fluid can damage the endocardium and permeate through the damaged endothelium of the cardiac wall and into the pericardial sac, leading to PCE/CT.
It takes an average of 3 to 4 days between catheter insertion and PCE diagnosis. According to a study analyzing 34 reports [3], PCE was diagnosed after an average of 4.0 days after UVC insertion, and an average of 3.64 days after PICC insertion. In our case, however, PCE and CT developed 29 hours after the initial UVC insertion. In addition, most of the cases reported so far have been related to PCE/CT in premature infants, but it should be recognized that this may occur even in full-term infants weighing >3 kg. A high-dextrose-containing PCE fluid was drained from the pericardial cavities, no bacteria was detected in the culture test, and no recurrence of PCE was reported after UVC removal. The association of PCE with TPN through the UVC was suspected as the aspirated fluid had a high dextrose content. The UVC tip was within the cardiac outline as recorded in the initial chest X-ray in our reported case. It is presumed that the UVC catheter was first sub-optimally placed in the right atrium; subsequently, the catheter gradually migrated into the pulmonary vein and passed through the right ventricle under the influence of repetitive heartbeats. The repetitive physical irritation of the catheter tip and chemical irritation of the hyperosmolar TPN fluid damaged the endothelium of the cardiac wall, leading to PCE/CT. The second PCE probably progressed rapidly within 2 hours due to the rapid permeation of hyperosmotic TPN fluid through the damaged endothelium.
Verification of proper catheter tip placement is important, both at the first placement and during follow-up, as catheter migration can occur. The possibility of malpositioning of the catheter tip must be excluded using both chest radiography and echocardiography [14,15]. Although UVC malposition is the most common cause of complications, PCE can rarely occur even when the UVC has been properly positioned. Clinicians should always consider that proper positioning of the UVC is important to minimize the associated complications and that complications cannot always be avoided.
As the symptoms and signs of PCE/CT are related to the nonspecific cardiopulmonary instability, the diagnosis and management of PCE/CT can be delayed, and late diagnosis and management can be fatal, even leading to death. Because PCE/CT could be caused by the presence of an in-dwelling CVC, sudden cardiopulmonary decompensation and rapidly progressing cardiomegaly in a patient with a UVC, should raise the suspicion of a possible PCE/CT; a diagnostic echocardiography should be performed immediately [2,16].
PCE/CT could be caused by CVC-related factors. Hence, regular verification of proper catheter tip placement using chest radiography and echocardiography is important, as early diagnosis and management of PCE/CT can be life-saving.
Notes
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (IRB) of Hanyang University Hospital, Seoul, Korea (IRB No. 2021-01-022). Written informed consent by the patients was waived due to a retrospective nature of our study.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conception or design: M.J.P., J.H.A., H.J.L., C.R.K., J.Y.N.
Acquisition, analysis, or interpretation of data: M.J.P., J.H.A.
Drafting the work or revising: M.J.P., H.K.P., J.K.H., J.Y.N.
Final approval of the manuscript: All authors read and approved the final manuscript.
Funding
None
Acknowledgements
None